Abstract
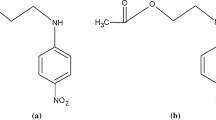
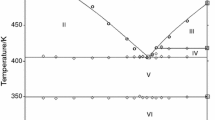
The solid–liquid equilibria (SLE) of two binary mixtures of organic stabilizers for energetic materials, viz. N-(2-methoxyethyl)-p-nitroaniline + 2-nitrodiphenylamine (S1) and N-(2-methoxyethyl)-p-nitroaniline + 1,3-diethyl-1,3-diphenylurea (S2), have been determined using differential scanning calorimetry at three heating rates β (0.5, 1, and 2 K min−1). The liquidus line has been predicted using four characteristic points of the mixture melting curve as follows: the maximum peak temperature (Ttop), the temperature proposed by the German Society of Thermal Analysis (TGEFTA), the inflection point temperature (Tinf), and the endset temperature (Tendset) obtained at β= 1 K min−1 and by extrapolating the three heat flow curves at β= 0 K min−1. The melting temperatures of pure compounds and the eutectics have been identified from the onset temperature obtained at β= 0 K min−1. The quality of the prediction has been evaluated by the computation of the global quality factor from SLE consistency tests and by the estimation of the uncertainty associated with each investigated temperature. It was found that the inflection point displays the highest quality factors and the lowest uncertainties. Moreover, and for practical purposes, the inflection temperature obtained at β= 1 K min−1 generated more consistent data and lowest uncertainties than the other temperatures obtained even at β= 0 K min−1.





Similar content being viewed by others
References
Oakley JH, Hughes TJ, Graham BF, Marsh KN, May EF. Determination of melting temperatures in hydrocarbon mixtures by differential scanning calorimetry. J Chem Thermodyn. 2017;108:59–70.
Leitner J, Jurik S. DSC study and thermodynamic modelling of the system paracetamol–o-acetylsalicylic acid. J Therm Anal Calorim. 2017;130(3):1735–40.
Chelouche S, Trache D, Neves CM, Pinho SP, Khimeche K, Benziane M. Solid + liquid equilibria and molecular structure studies of binary mixtures for nitrate ester’s stabilizers: measurement and modeling. Thermochim Acta. 2018;666:197–207.
Silva LPS, Dalmazzone D, Stambouli M, Lesort A-L, Arpentinier P, Trueba A, et al. Phase equilibria of semi-clathrate hydrates of tetra-n-butyl phosphonium bromide at atmospheric pressure and in presence of CH4 and CO2 + CH4. Fluid Phase Equilib. 2016;413:28–35.
Diarce G, Gandarias I, Campos-Celador A, García-Romero A, Griesser U. Eutectic mixtures of sugar alcohols for thermal energy storage in the 50–90 C temperature range. Sol Energy Mater Sol Cells. 2015;134:215–26.
Wang Y, Wang J, Zhao X, Zhu L, Yang L, Sha Z. Determination and thermodynamic modeling of solid–liquid phase equilibrium for the 2,4,6-trimethylphenol and 2,5-dimethylphenol binary system. J Therm Anal Calorim. 2018;132(3):1923–31.
Marinescu D-C, Pincu E, Stanculescu I, Meltzer V. Thermal and spectral characterization of a binary mixture (acyclovir and fluocinolone acetonide): eutectic reaction and inclusion complexes with β-cyclodextrin. Thermochim Acta. 2013;560:104–11.
Okuniewski M, Paduszyński K, Domańska U. Phase diagrams in representative terpenoid systems: measurements and calculations with leading thermodynamic models. Ind Eng Chem Res. 2017;56(34):9753–61.
Bessa LCBA, Robustillo MD, de Almeida Meirelles AJ, de Alcântara Pessôa Filho P. (Solid + liquid) equilibrium of binary mixtures containing ethyl esters and p-xylene by differential scanning calorimetry. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08085-z
Kousksou T, Jamil A, El Rhafiki T, Zeraouli Y. Paraffin wax mixtures as phase change materials. Sol Energy Mater Sol Cells. 2010;94(12):2158–65.
Takiyama H, Suzuki H, Uchida H, Matsuoka M. Determination of solid–liquid phase equilibria by using measured DSC curves. Fluid Phase Equilib. 2002;194:1107–17.
Huang C-C, Chen Y-P. Measurements and model prediction of the solid–liquid equilibria of organic binary mixtures. Chem Eng Sci. 2000;55(16):3175–85.
Lin W, Dalmazzone D, Fürst W, Delahaye A, Fournaison L, Clain P. Accurate DSC measurement of the phase transition temperature in the TBPB–water system. J Chem Thermodyn. 2013;61:132–7.
Höhne G, Cammenga H, Eysel W, Gmelin E, Hemminger W. The temperature calibration of scanning calorimeters. Thermochim Acta. 1990;160(1):1–12.
Lin W, Dalmazzone D, Fürst W, Delahaye A, Fournaison L, Clain P. Thermodynamic properties of semiclathrate hydrates formed from the TBAB + TBPB + water and CO2 + TBAB + TBPB + water systems. Fluid Phase Equilib. 2014;372:63–8.
Mayoufi N, Dalmazzone D, Delahaye A, Clain P, Fournaison L, Fürst W. Experimental data on phase behavior of simple tetrabutylphosphonium bromide (TBPB) and mixed CO2 + TBPB semiclathrate hydrates. J Chem Eng Data. 2011;56(6):2987–93.
Paunovic I, Mehrotra AK. Liquid–solid phase transformation of C16H34, C28H58 and C41H84 and their binary and ternary mixtures. Thermochim Acta. 2000;356(1–2):27–38.
Hammami A, Mehrotra AK. Liquid–solid–solid thermal behaviour of n-C44H90 + n-C50H102 and n-C25H52 + n-C28H58 paraffinic binary mixtures. Fluid Phase Equilib. 1995;111(2):253–72.
Chelouche S, Trache D, Tarchoun AF, Abdelaziz A, Khimeche K, Mezroua A. Organic eutectic mixture as efficient stabilizer for nitrocellulose: kinetic modeling and stability assessment. Thermochim Acta. 2019;673:78–91.
Trache D, Tarchoun AF. Analytical methods for stability assessment of nitrate esters-based propellants. Crit Rev Anal Chem. 2019. https://doi.org/10.1080/10408347.2018.1540921
Trache D, Tarchoun AF. Stabilizers for nitrate ester-based energetic materials and their mechanism of action: a state-of-the-art review. J Mater Sci. 2018;53(1):100–23.
Gibson JD. Stabilizers for cross-linked composite modified double base propellants. US Patent 5,387,295; 1995.
Trache D, Khimeche K, Benziane M, Dahmani A. Solid–liquid phase equilibria for binary mixtures of propellant’s stabilizers. J Therm Anal Calorim. 2013;112(1):215–22.
Lide DR. CRC handbook of chemistry and physics. Boca Raton: CRC; 2012.
Trache D, Khimeche K, Benelmir R, Dahmani A. DSC measurement and prediction of phase diagrams for binary mixtures of energetic materials’ stabilizers. Thermochim Acta. 2013;565:8–16.
Książczak A, Książczak T, Ostrowski M. Intermolecular interactions and phase equilibria in nitrocellulose-s-diethyldiphenylurea system. J Therm Anal Calorim. 2003;74(2):575–81.
Keshavarz MH, Akbarzadeh AR, Rahimi R, Jafari M, Pasandideh M, Sadeghi R. A reliable method for prediction of enthalpy of fusion in energetic materials using their molecular structures. Fluid Phase Equilib. 2016;427:46–55.
Jain A, Yang G, Yalkowsky SH. Estimation of melting points of organic compounds. Ind Eng Chem Res. 2004;43(23):7618–21.
Chelouche S, Trache D, Pinho SP, Khimeche K, Mezroua A, Benziane M. Solid–liquid phase equilibria, molecular interaction and microstructural studies on (N-(2-ethanol)-p-nitroaniline + N-(2-acetoxyethyl)-p-nitroaniline) binary mixtures. Int J Thermophys. 2018;39(11):129.
Saeed RM, Schlegel J, Castano C, Sawafta R. Uncertainty of thermal characterization of phase change material by differential scanning calorimetry analysis. Int J Eng Res Technol. 2016;5(1):405–12.
He B, Martin V, Setterwall F. Liquid–solid phase equilibrium study of tetradecane and hexadecane binary mixtures as phase change materials (PCMs) for comfort cooling storage. Fluid Phase Equilib. 2003;212(1–2):97–109.
Kang JW, Diky V, Chirico RD, Magee JW, Muzny CD, Kazakov AF, et al. Algorithmic framework for quality assessment of phase equilibrium data. J Chem Eng Data. 2014;59(7):2283–93.
Chabane S, Benziane M, Khimeche K, Trache D, Didaoui S, Yagoubi N. Low-temperature behavior of diesel/biodiesel blends. J Therm Anal Calorim. 2018;131(2):1615–24.
Rajan A, Kuang YC, Ooi MP-L, Demidenko S, editors. Standard uncertainty estimation on polynomial regression models. In: IEEE Sensors Applications Symposium, Queenstown, New Zealand; 2014.
Gelman A, Imbens G. Why high-order polynomials should not be used in regression discontinuity designs. J Bus Econ Stat. 2018. https://doi.org/10.1080/07350015.2017.1366909
Campanella L, Micieli V, Tomassetti M, Vecchio S. Solid–liquid phase diagrams of binary mixtures. J Therm Anal Calorim. 2010;99(3):887–92.
Hernandez FR, Djurdjevic M, Kierkus W, Sokolowski J. Calculation of the liquidus temperature for hypo and hypereutectic aluminum silicon alloys. Mater Sci Eng A. 2005;396(1–2):271–6.
Acknowledgements
The authors are grateful for the financial support of this research from Ecole Militaire Polytechnique (Doctoral Training Program).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chelouche, S., Trache, D. & Khimeche, K. Suitable temperature assignment for liquidus line in SLE investigation by DSC. J Therm Anal Calorim 139, 475–487 (2020). https://doi.org/10.1007/s10973-019-08392-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08392-5