Abstract
A new compound of the formula Mg3In4V6O24 belonging to the family M3A4V6O24 [where M = metal(II), A = metal(III)] was obtained by the solid-state reaction method. The compound was synthesized by heating in air a mixture of Mg3(VO4)2 and InVO4 or MgV2O6 and In2O3 or In2O3 and V2O5, with a precursor of MgO. The new compound was characterized by XRD, SEM, IR, UV–Vis and DTA–TG methods. Mg3In4V6O24 was shown to crystallize in the triclinic system. Its unit cell parameters were calculated. The new compound is stable in air up to 1000 °C and is a semiconductor (Eg ~ 3.3 eV).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Semiconductors have been for a long time fundamental to development of industry and are pivotal elements of many devices. Ceramic materials based on indium(III) orthovanadate(V) have been used for production of anodes because of their electrochemical stability and high electric capacity [1,2,3]. Recently, also InVO4 admixtured with divalent metals has been found to be a suitable catalyst in the process of photocatalytic decomposition of water [4, 5].
The choice of a compound from the family M3A4(VO4)6 as the subject of the study was dictated first of all by the structure of this family compounds and interesting application possibilities of known vanadates(V) of divalent metals and indium(III) orthovanadates(V).
Literature data imply the existence of a series of compounds of the general formula M3Fe4(VO4)6 in the three-component metal oxide systems of MO–Fe2O3–V2O5 (M = Zn, Mg, Mn, Cu) [6,7,8,9,10]. What is more, also the compound of the formula M2FeV3O11 has been reported to be formed in some of these systems [11,12,13]. The structures of Mn3Fe4(VO4)6 and Cu3Fe4(VO4)6 are known [7,8,9, 14]. These compounds crystallize in the triclinic system [7,8,9].
To the best of our knowledge, the ternary oxide system, i.e., MgO–In2O3–V2O5, has been previously examined to a very limited extend. According to the hitherto published scarce data on this system, as a result of the reaction of Mg2V2O7 with InVO4 in solid state, one compound Mg2InV3O11 is formed [15]. The similarity of unit cell parameters of Mg2InV3O11 and those of Mg2FeV3O11 as well as the similarity of their IR spectra indicates that they are isostructural [15].
The ternary oxide systems MO–In2O3–V2O5, where M = Co, Cd, Cu, Ni, Pb, Sr have been already studied [16,17,18,19,20,21,22]. Results of the studies revealed the formation of a series of vanadates: M2InV3O11 (M = Co, Pb, Sr) [17, 19, 22], but only in the system CdO–In2O3–V2O5 a compound of the formula Cd3In4V6O24 was formed [18]. The compound crystallizes in the monoclinic system, and it has not been isostructural with the family of compounds of the general formula M3A4(VO4)6.
The main aim of the presented work was to check whether in the system Mg3(VO4)–InVO4 which is one of the cross-sections of the system MgO–In2O3–V2O5, any new compounds are formed and if so, to determine its some physicochemical properties.
Experimental
The commercial reagents used for syntheses were:
3MgCO3·Mg(OH)2·3H2O (a.p., POCh, Gliwice, Poland), as a precursor of MgO,
In2O3 (a.p., Aldrich, Germany),
V2O5 (a.p., Riedel-de Haën, Germany).
Also, InVO4, MgV2O6 and Mg3(VO4)2 were used as reagents in the conducted studies, and their syntheses are reported in [16, 23, 24]. The syntheses were carried out by the standard method of calcinations, described, e.g., in paper [25]. The reacting substances were weighed in appropriate portions, thoroughly homogenized by grinding, shaped into pellets and heated in cycles in a syllite furnace, in air. After each heating stage the samples were cooled together with the furnace to ambient temperature, ground and subjected to DTA and powder X-ray diffraction (XRD) measurements. After shaping them again into pellets, they were further heated. This procedure was repeated until equilibrium samples were obtained.
The types of phases occurring in particular samples were determined with the use of XRD method. Powder X-ray diffraction patterns of the samples were recorded with a PANalytical Empyrean II diffractometer in the 2Θ range of 10°–60° with graphite monochromator and CuKα1,2 radiation (λ = 0.15418 nm) within the continuous scanning mode with a step of 0.013°, generator voltage 40 kV, tube current 30 mA and divergence slit 1.0°. The phases were identified on the basis of X-ray characteristics contained in PDF cards [26] and found in the literature [15].
The DTA-Tg measurements were conducted by using a derivatograph Q-1500 of Paulik-Paulik-Erdey (MOM, Budapest, Hungary). The samples to be examined by this method, in portions of 500 mg, were placed in corundum crucibles. All measurements were performed in air, within the temperature range 20–1300 °C, at a heating rate of 10° min−1.
The density of the new compound was determined using a gas pycnometer Ultrapyc 1200e (Quantachrome Instruments USA) using argon of 5 N purity as pycnometric gas.
The unit cell parameters were calculated with the aid of the program POWDER [27] belonging to the crystallographic program library X-ray System 70. Accurate positions of the diffraction lines were established by the internal standard method. The internal standard was α-Al2O3. The parameters of the unit cell were refined by the Refinement program of DHN/PDS package.
The infrared spectra were recorded in the wavenumber range 1100–250 cm−1 using a spectrometer SPECORD M 80 (Carl Zeiss, Jena, Germany). The measurements were performed by mixing the investigated sample with KBr at the mass ratio of 1:300.
A sample of the new compound was examined using scanning electron microscope (JSM-1600, Joel, Japan).
Optical absorption spectra of new phase were recorded at room temperature on UV–Vis–NIR JASCO-V670 spectrophotometer in the wavelength region of 180–1000 nm. The spectrophotometer was equipped with an integrating sphere assembly and BaSO4 was a reference.
Results and discussion
Twelve samples were prepared from V2O5, In2O3 and 3MgCO3·Mg(OH)2·3H2O with compositions representing the whole range of the components concentrations of Mg3(VO4)–InVO4 system. The compositions of all samples, in terms of the contents of Mg3(VO4)2 and InVO4, as well as oxides are shown in Table 1, which also gives the compositions of the initial mixtures of the samples, conditions of their heating and the phases detected after the last stage of heating.
Taking into account that the new set of XRD lines was recorded in the diffractogram of the sample whose initial mixture with V2O5 and In2O3 contained 37.50 mol % of MgO (Table 1, sample 10), the new compound was described by the stoichiometric formula Mg3In4V6O24. The diffraction pattern of the new compound contained, however, a set of diffraction lines at positions close to those of the lines characteristic of Mg3Fe4V6O24 [6]. It means that the new compound belongs to the family of the general formula M3A4(VO4)6 [where M = metal(II), A = metal(III)]. The new compound of the formula Mg3In4V6O24 was obtained as a result of the solid-state reaction:
The new compound Mg3In4V6O24 was obtained also as a result of the solid-state reaction between Mg3(VO4)2 and InVO4 mixed at the molar ratio 1:4 or MgV2O6 and indium(III) oxide, mixed at the molar ratio 3:2, heated in the following stages: 700 °C(12 h) + 850 °C(12 h) + 950 °C(12 h × 2):
Mg3In4V6O24 has a light yellow color. Figure 1 presents fragments of the diffractogram of a mixture of MgV2O6 and In2O3 (Fig. 1b) as well as the diffractogram of the new compound Mg3In4V6O24 (Fig. 1a).
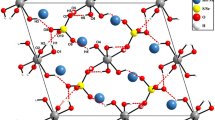
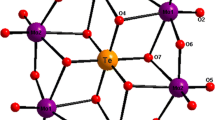
The powder diffractograms of Mg3In4V6O24 was subjected to indexation, and the results are displayed in Table 2. The parameters of its triclinic elementary cell are: a = 0.6649(8) nm, b = 0.8231(4) nm, c = 1.0349(8) nm, α = 103.06°, β = 103.45° γ = 102.75°, Z = 1; the X-ray density is drtg = 3.95 g cm−3, while the pycnometric density is dpic = 4.00 ± 0.05 g cm−3. The results of this part of the study revealed that Mg3In4V6O24 was isostructural with Mg3Fe4V6O24 [6]. A SEM image of Mg3In4V6O24 (Fig. 2) shows the presence of only one crystals’ habit. The crystals of the new compound have different sizes, from larger ones of 4 µm to small ones of about 1 µm.
IR spectrum of Mg3In4V6O24 is shown in Fig. 3. The spectrum is very similar to that of Mg3Fe4V6O24 which is shown and described in [6]. Three ranges of absorption bands can be distinguished in the spectrum of Mg3In4V6O24 (Fig. 3). The first one between 1050 and 880 cm−1 exhibits two distinct maxima occurring at 960 and 930 cm−1. In this wavenumber range, the absorption is caused by the stretching vibrations of V–O bonds in the VO4 tetrahedra [3, 28]. The second wide absorption band, in the wavenumber range 880–570 cm−1, exhibits absorption maxima at 820 and 750 cm−1. This band can be ascribed to stretching vibrations of the In–O bonds in InO6 [29, 30]. In the last wavenumber range, i.e., 520–280 cm−1, the band can be assigned to the stretching vibrations of Mg–O bonds in the MgO6 octahedra [11, 31] as well as to the bending vibrations of the O–V–O bonds in the VO4 tetrahedra. It cannot be excluded that the absorption in this wavenumber range has a combined nature.
The DTA curve of Mg3In4V6O24 up to 1350 °C reveals only one endothermic effect with its onset temperature equal to 1000 ± 5 °C (Fig. 4). In order to determine the kind of process beginning at this temperature, monophasic sample containing Mg3In4V6O24 was heated at 1050 °C for 1.5 h and subsequently cooled down rapidly to room temperature (at the moment of removing the sample from the furnace it was melted) and subjected to tests by XRD method. Phase composition analysis of this sample showed the presence of In2O3 (PDF card no. 06-0416) as well as a small amount of Mg3In4V6O24. The obtained results indicate that the endothermic effect recorded in the DTA curve is due to incongruent melting of Mg3In4V6O24 in accordance with the reaction:
In the next step, the new compound Mg3In4V6O24 was investigated using UV–Vis–NIR method. Figure 5 shows the optical absorption spectra of Mg3In4V6O24 at room temperature recorded between 180 and 1000 nm. The spectrum permitted determination of the energy gap for the compound. The approximate value of Eg was found according to the Kubelka–Munk transformation. The energy gap determined in this way for Mg3In4V6O24 was Eg ~ 3.3 eV, which means that this compound is semiconductor.
Conclusions
The results obtained in the study have shown that in the ternary oxide system MgO–In2O3–V2O5 a new compound of the formula Mg3In4V6O24 is formed. This new compound was obtained as a result of the reaction in solid state between the oxides In2O3 and V2O5 with the precursor of magnesium oxide, i.e., with 3MgCO3·Mg(OH)2·3H2O, between Mg3(VO4)2 and InVO4 or MgV2O6 with In2O3. Mg3In4V6O24 crystallizes in the triclinic system with the unit cell parameters: a = 0.6649(8) nm, b = 0.8231(4) nm, c = 1.0349(8) nm, α = 103.06°, β = 103.45° γ = 102.75°, Z = 1 and it is isostructural to Mg3Fe4V6O24. The new compound has a light yellow color. It melts incongruently at 1000 ± 5 °C with deposition of solid In2O3. On the basis of the energy gap calculated for Mg3In4V6O24 (Eg ~ 3.3 eV), it was classified as a semiconductor.
References
Denis S, Baudrin E, Touboul M, Tarascon JM. Synthesis and electrochemical properties of amorphous vanadates of general formula RVO4 (R = In, Cr, Fe, Al, Y). J Electrochem Soc. 1997;144:4099–109.
Duan F, Wang J, Chen M. Shape-controllable synthesis and electrochemical performance of InVO4 microstructures as electrode materials for Li-ion battery. J Nano Res. 2013;23:108–12.
Orel B, Šurca Vuk A, Opara Krašovec U, Dražič G. Electrochromic and structural investigation of InVO4 and some other vanadia-based films. Electrochim Acta. 2001;46:2059–68.
Yan Y, Liu X, Fan W, Lv P, Shi W. InVO4 microspheres: preparation, characterization and visible-light-driven photocatalytic activities. Chem Eng J. 2012;200–202:310–6.
Li GL, Yin Z. Theoretical insight into electronic, optical and photocatalytical properties of InMO4 (M = V, Nb, Ta) photocatalysts. Phys Chem Chem Phys. 2011;13:2824–33.
Kurzawa M, Blonska-Tabero A. The synthesis and selected properties of new compounds: Mg3Fe4(VO4)6 and Zn3Fe4(VO4)6. Mater Res Bull. 2002;37:849–58.
Belik AA, Malakho AP, Pokholok KV, Lazoryak BI. Phase formation in Cu3+1.5xR4−x(VO4)6 (R = Fe and Cr) systems: crystal structure of Cu2.5Fe4.333(VO4)6, Cu4.05Cr3.3(VO4)6. J Solid State Chem. 2001;156:339–48.
Wang X, Vander Griend DA, Stern CL, Poeppelmeier KR. Site-specific vanadates Co4Fe3.33(VO4)6 and Mn3Fe4(VO4)6. Inorg Chem. 2000;39:136–40.
Lafontaine MA, Greneche JM, Laligant Y, Ferey G. β-Cu3Fe4(VO4)6: structural study and relationships; physical properties. J Solid State Chem. 1994;208:1–10.
Zolnierkiewicz G, Guskos N, Typek J, Anagnostakis EA, Blonska-Tabero A, Bosacka M. Competition of magnetic interactions in M3Fe4V6O24 (M(II) = Zn, Cu, Mn, Mg) compounds studied by EPR. J Alloys Compd. 2009;471:28–32.
Wang X, Vander Griend DA, Stern ChL. Poeppelmeier KR Structure and cation distribution of new ternary vanadates FeMg2V3O11 and FeZn2V3O11. J Alloys Compd. 2000;298:119–24.
Kurzawa M, Blonska-Tabero A, Rychlowska-Himmel I. Phase relations in subsolidus area of ZnO–V2O5–Fe2O3 system. J Therm Anal Calorim. 2003;74:537–42.
Tabero P, Blonska-Tabero A, Szilágyi PA, Homonnaty Z. The investigations of phases with general formula M2FeV3O11, where M = Mg Co, Ni, Zn by IR and Mössbauer spectroscopy. J Phys Chem Solids. 2007;68:1087–90.
Hughes JM, Starkey SJ, Malinconico ML, Malinconico LL. Lyonsite, Cu 2+3 Fe4 3+(VO4)6, a new fumarolic sublimate from Izalco volcano, El Salvador: descriptive mineralogy and crystal structure. Am Mineral. 1988;73:181–6.
Bosacka M. The synthesis and selected properties of new double vanadates M2InV3O11, where M = Zn, Mg. Mater Res Bull. 2006;41:2181–6.
Bosacka M. The synthesis and selected properties of Co2InV3O11. J Therm Anal Calorim. 2007;88:43–6.
Bosacka M. The subsolidus area of CoO–In2O3–V2O5 system. J Therm Anal Calorim. 2012;109:511–5.
Bosacka M. Phase diagram of CdO–V2O5–In2O3 system. J Alloys Compd. 2013;574:266–71.
Filipek E, Paczesna A, Piz M. Sr2InV3O11—new ceramic compound in Sr2V2O7–InVO4 system and its characteristic. Ceram Int. 2016;42:14148–54.
Paczesna A, Filipek E. Characterization of new compound and phase equilibria up to solidus line in the system InVO4–NiO. Thermochim Acta. 2015;618:67–73.
Bosacka M, Filipek E, Paczesna A. Unknown phase equilibria in the ternary oxide V2O5–CuO–In2O3 system in subsolidus area. J Therm Anal Calorim. 2016;125:1161–70.
Blonska-Tabero A, Bosacka M. Comparative studies in subsolidus areas of ternary oxide systems PbO–V2O5–In2O3 and PbO–V2O5–Fe2O3. J Therm Anal Calorim. 2013;113:137–45.
Kurzawa M, Rychlowska-Himmel I, Blonska-Tabero A, Bosacka M, Dąbrowska G. A new compound Mg2CrV3O11 and phase relation in the MgV2O6–MgCr2O4 system in solid state. Solid State Phenom. 2003;90–91:353–8.
Wang D, Tang J, Zou Z, Ye J. Photophysical and photocatalytic properties of a new series of visible-light-driven photocatalysts M3V2O8 (M = Mg, Ni, Zn). Chem Mater. 2005;17:5177–82.
Blonska-Tabero A, Bosacka M, Dąbrowska G, Filipek E, Piz M, Rychlowska-Himmel I, Tabero P, Tomaszewicz E. The synthesis and properties of the phases obtained by solid–solid reaction. J Min Metall. 2008;44B:19–26.
Powder Difraktion File, International Center for Diffraction Data, Swarthmore (USA), File Nos.: 04-0829, 06-0416, 09-0387, 31-0816, 33-0628, 36-0309, 36-0310, 37-0351, 70-1163.
Taupin D. A powder–diagram automatic–indexing routine. J Appl Crystallogr. 1973;6:380–5.
Cimino N, Artuso F, Decker F, Orel B, Šurca Vuk A, Zanoni R. XPS and IR studies of transparent InVO4 films upon Li charge–discharge reactions. Solis State Ionics. 2003;165:89–96.
Tenailleau C, Pring A, Moussa SM, Liu Y, Withers RL, Tarantino S, Zhang M, Carpenter MA. Composition-induced structural phase transition in the (Ba1−xLax)2In2O5+x (0 ≤ x≤0.6) system. J Solid State Chem. 2005;178:882–91.
Cai G, Chen XL, Wang WY, Lou YF, Liu J, Zhao JT, Chen HH. A new promising scintillator Ba3InB9O18. J Solid State Chem. 2008;181:646–51.
Müller C, Müller-Buschbaum H. Zu kenntnis Mg2−xZnxGaV3O11 (x = 0.3). J Alloys Compd. 1992;185:163–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bosacka, M. New compound Mg3In4V6O24 and its physicochemical characteristic. J Therm Anal Calorim 138, 4289–4294 (2019). https://doi.org/10.1007/s10973-019-08122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08122-x