Abstract
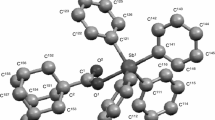
In the present research, the temperature dependence of heat capacity of tris(pentafluorophenyl)-4-pyridylethylgermane (C6F5)3Ge–CH2–CH2–C5H4N was studied by precise adiabatic vacuum calorimetry and differential scanning calorimetry over the temperature range from 6 to 450 K. The temperature and enthalpy of fusion of tris(pentafluorophenyl)-4-pyridylethylgermane and the total mole fraction of impurities have been determined. The thermal stability of the sample was investigated by thermogravimetric analysis. The experimental data were used to calculate the standard thermodynamic functions: heat capacity, enthalpy, entropy, and the Gibbs energy over the range from T → 0 to 420 K for crystalline and liquid states. For the compound under study, the standard entropy of formation in the crystalline state was calculated at T = 298.15 K. In addition, the structure of the investigated compound was established, and corresponding structural parameters were determined.





Similar content being viewed by others
References
Chambers RD. Fluorine in organic chemistry. Oxford: Blackwell Publishing Ltd.; 2004.
Bardin VV. Synthesis of bis(pentafluorophenyl)phenylhalogenogermanes and bis(pentafluorophenyl)dihalogenogermanes, (C6F5)2GeXY (X = Ph, Y = Br, Cl; X = Y = F, Cl, Br). J Organomet Chem. 2016;822:46–52.
Song B, Yang S, Zhong H, Jin L, Hu D, Liu G. Synthesis and bioactivity of 2-cyanoacrylates containing a trifluoromethylphenyl moiety. J Fluor Chem. 2005;126:7–92.
Banks RE, Smart BE, Tatlow JC. Organofluorine. Chemistry principles and commercial applications. New York: Plenum; 1994.
Kissa E. Fluorinated surfactants: synthesis, properties, applications. Surfactant Science Series, Vol. 50. New York: Marcel Dekker; 1994.
Kronberg B, Holmberg K, Lindman B. Surface chemistry of surfactants and polymers. Chichester: Wiley; 2014.
Guo W, Brown TA, Fung BM. Micelles and aggregates of fluorinated surfactants. J Phys Chem. 1991;95:1829–36.
Magueur G, Crousse B, Charneau S, Grellier P, Begue JP, Bonnet-Delpon D. Fluoroartemisinin: trifluoromethyl analogues of artemether and artesunate. J Med Chem. 2004;47:2694–9.
Gamage SA, Spicer JA, Rewcastle GW, Milton J, Sohal S, Dangerfield W, Mistry P, Vicker N, Charlton PA, Denny WA. Structure-activity relationships for pyrido-, imidazo-, pyrazolo-, pyrazino-, and pyrrolophenazinecarboxamides as topoisomerase-targeted anticancer agents. J Med Chem. 2002;45:740–3.
Abdou IM, Saleh AM, Zohdi HF. Synthesis and antitumor activity of 5-trifluoromethyl-2,4- dihydropyrazol-3-one nucleosides. Molecules. 2004;9:109–16.
Filler R. Biochemistry involving carbon–fluorine bonds. Washington: American Chemical Society; 1976.
Bhamaria RP, Bellare RA, Deliwala CV. In intro effect of 1-acyl-4-alkyl-(oraryl)-thiosemicarbazides 1-(5-chlorosalicylidine)-4-alkyl-(oraryl)-thiosemicarbazones and some hydrazones of 5-chlorosalicylaldehyde against pathogenic bacteria including mycobacterium tuberculosis (H37Rv). Indian J Exp Biol. 1968;6:62–3.
Fujiwara T, Takeuchi Y. Synthesis, reactions, and applications of fluorine-containing multifunctional carbon compounds. J Fluor Chem. 2005;126:941–55.
Salunkhe NG. Green synthesis, characterization and biological evaluation of some triazole and thiadiazole. J Curr Chem Pharm Sci. 2012;2(2):100–6.
Ishikawa N. Synthesis function of fluorinated compounds. CMC: Tokyo; 1987.
Zamyshlyaeva OG, Lapteva OS, Blinova LS, Fukin GK. Tris-(pentafluorophenyl)-4-pyridylethylgermane and method for production thereof. Patent RU 2591958 C1; 2016.
Gasilova ER, Saprykina NN, Zamyshlyayeva OG, Semchikov YuD, Bochkarev MN. Hyperbranched perfluorinated poly(phenylenegermanes) obtained by polycondensation of A2B2 and AB3 monomers. J Phys Org Chem. 2010;23:1099–107.
SAINT. Data reduction and correction program., v. 8.34A and v.8.37A, Bruker AXS, Madison, Wisconsin, USA. 2014.
Sheldrick GM. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015;C71:3–8.
Sheldrick GM. SADABS 2012/1, Bruker AXS area detector scaling and absorption correction. Madison: Bruker AXS; 1998.
Piskunov AV, Aivaz’yan IA, Poddel’sky AI, Fukin GK, Baranov EV, Cherkasov VK, Abakumov GA. New germanium complexes containing ligands based on 4,6-di-tert-butyl-N-(2,6-diisopropylphenyl)-o-iminobenzoquinone in different redox states. Eur J Inorg Chem. 2008;8:1435–44.
Uhl W, Bohnemann J, Kappelt B, Malessa K, Rohling M, Tannert J, Layh M, Hepp A. Hydrometallation (M = Al, Ga) of silicon- and germanium-centred oligoalkynes. Z Naturforsch. 2014;69b:1333–47.
Rohwer H, Dillen J. Deviation from tetrahedral geometry in Me2GeCl2: crystal structure of a model compound and insight from ab initio calculations. Inorg Chem. 2002;41:4167–72.
Batsanov SS. Van der Waals radii of elements. Inorg Mater. 2001;37:871–85.
Varushchenko RM, Druzhinina AI, Sorkin EL. Low-temperature heat capacity of 1-bromoperfluorooctane. J Chem Thermodyn. 1997;29:623–7.
Malyshev VM, Milner GA, Sorkin EL, Shibakin VF. Automatic low-temperature calorimeter. Prib Tekh Eksp. 1985;6:195–7 [in Russian].
Höhne GWH, Hemminger WF, Flammersheim H-J. Differential scanning calorimetry. 2nd ed. Heidelberg: Springer; 2003.
Drebushchak VA. Calibration coefficients of heat-flow DSC. Part II. Optimal calibration procedure. J Therm Anal Calorim. 2005;79:213–8.
Aleksandrov YuI. Tochnaya Kriometriya Organicheskih Veshestv. Leningrad: Khimiya; 1975.
Rabinovich IB, Nistratov VP, Telnoy VI, Sheiman MS. Thermochemical and thermodynamic properties of organometallic compounds. New York: Begell House Inc., Publishers; 1999.
Sologubov SS, Markin AV, Smirnova NN, Rybakova YuA, Novozhilova NA, Tatarinova EA, Muzafarov AM. Calorimetric study of carbosilane dendrimers of the third and sixth generations with phenylethyl terminal groups. J Therm Anal Calorim. 2016;125:595–606.
McCullough JP, Scott DW. Calorimetry of non-reacting systems. London: Butterworth; 1968.
Chase MW Jr. NIST-JANAF thermochemical tables, 4th ed. J Phys Chem Ref Data Monogr. 1998;9:1951. http://webbook.nist.gov/chemistry/. Accessed 5 July 2018.
Cox JD, Wagman DD, Medvedev VA. CODATA key values for thermodynamics. New York; 1989. http://webbook.nist.gov/chemistry/. Accessed 5 July 2018.
Acknowledgments
This work was performed with the financial support of the Ministry of Education and Science of the Russian Federation (Contracts Nos. 4.6138.2017/6.7 and 4.5706.2017/8.9) and the Russian State Assignment (Theme No. 44.2, Reg. No. AAAA-A16-116122110053-1). The elemental analysis was conducted based on equipment of the Common Use Center « New Materials and Resource-saving Technologies» of the Research Institute for Chemistry of National Research Lobachevsky State University of Nizhny Novgorod.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamyshlyayeva, O.G., Markin, A.V., Smirnova, N.N. et al. Calorimetric and structural studies of organic compound of tris(pentafluorophenyl)-4-pyridylethylgermane. J Therm Anal Calorim 136, 1227–1236 (2019). https://doi.org/10.1007/s10973-018-7786-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7786-6