Abstract
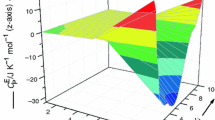
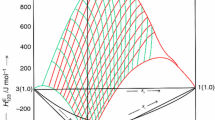
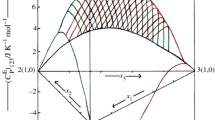
In recent years, studies on mixtures consisting of ionic liquids and organic solvents have gained importance for the application of such mixtures for new chemical processes and technologies in industries. In this contribution, new experimental excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) data of ternary 1-butyl-2,3-dimethylimidazolium tetrafluoroborate, [Bmmim][BF4] (i) + 1-butyl-3-methylimidazolium tetrafluoroborate, [Bmim][BF4] or 1-ethyl-3-methylimidazolium tetrafluoroborate, [Emim][BF4] (j) + cyclopentanone (CPO) or cyclohexanone (CHO) (k) mixtures, have been reported over the whole composition range at 298.15 K and atmospheric pressure. The observed data have been satisfactorily correlated by Redlich–Kister equation for each mixture. The \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CPO (k) mixtures are positive over whole range of composition of xi and xj. The sign and magnitude of \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CHO (k) mixtures vary with the change in composition of the components of the mixtures. The \( H_{\text{ijk}}^{\text{E}} \) data have also been analyzed in terms of graph theory (which involves the topology of the molecule). It has been observed that estimated values by graph theory compare well with their corresponding experimental values.






Similar content being viewed by others
References
Nebig S, Bölts R, Gmehling J. Measurement of vapor–liquid equilibria (VLE) and excess enthalpies (H E) of binary systems with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and prediction of these properties and γ using modified UNIFAC (Dortmund). Fluid Phase Equilib. 2007;258:168–78.
Safarov J, Hassel E. Thermodynamic properties of 1-hexyl-3-methylimidazolium tetrafluoroborate. J Mol Liq. 2010;153:153–8.
Shamsipur M, Miran Beigi AA, Teymouri M, Pourmortazavi SM, Irandoust M. Physical and electrochemical properties of ionic liquids 1-ethyl-3- methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide. J Mol Liq. 2010;157:43–50.
Gaciño FM, Paredes X, Comuñas MJP, Fernández J. Pressure dependence on the viscosities of 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide and two tris(pentafluoroethyl)trifluorophosphate based ionic liquids: new measurements and modelling. J Chem Thermodyn. 2013;62:162–9.
Fan XH, Chen YP, Su CS. Density and Viscosity Measurements for binary mixtures of 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF4]) with dimethylacetamide, dimethylformamide, and dimethyl sulfoxide. J Chem Eng Data. 2016;61:920–7.
Shekaari H, Kazempour A. Solution properties of ternary d-glucose + 1-ethyl-3- methylimidazolium ethyl sulfate + water solutions at 298.15 K. J Solut Chem. 2011;40:1582–95.
Yang JZ, Guan W, Tong J, Wang H, Li L. Studies of thermochemical properties of a new ionic liquid prepared by mixing 1-methyl-3-pentylimidazolium chloride with InCl3. J Solut Chem. 2006;35:845–52.
Altuwaim MS, Alkhaldi KHAE, Al-Jimaz AS, Mohammad AA. Temperature dependence of physicochemical properties of imidazolium-, pyroldinium-, and phosphonium-based ionic liquids. J Chem Eng Data. 2014;59:1955–63.
Dai Y, Qu Y, Wang S, Wang J. Measurement, correlation, and prediction of vapor pressure for binary and ternary systems containing an alkylsulfate-based ionic liquid. Fluid Phase Equilib. 2015;397:58–67.
Chun Hua H, Sorianoa AN, Lerona RB, Hui Li M. Molar heat capacity of four aqueous ionic liquid mixtures. Thermochim Acta. 2011;519:44–9.
Shekaari H, Zafarani-Moattar MT, Mirheydari SN. Density, viscosity, speed of sound, and refractive index of a ternary solution of aspirin, 1-butyl-3-methylimidazolium bromide, and acetonitrile at different temperatures T = (288.15 to 318.15) K. J Chem Eng Data. 2015;60:1572–83.
Kim KI, Shin BK, Ziegler F. XV international symposium of thermophysical properties, Colorado, U.S.A; 2003.
Raeissia S, Petersb CJ. Density, viscosity, speed of sound, and refractive index of a ternary solution of aspirin, 1-butyl-3-methylimidazolium bromide, and acetonitrile at different temperatures T = (288.15 to 318.15) K. Fluid Phase Equilib. 2010;294:67–71.
Choraüzewski M, Tkaczyk M. Heat capacity, speed of ultrasound, and density for 1,5-dibromopentane + heptane within the temperature range from 293.15 K to 313.15 K. J Chem Eng Data. 2006;51:1825–31.
Oliveira MB, Domínguez-Pérez M, Cabeza O, Lopes-da-Silva JA, Freire MG, Coutinho JAP. Surface tensions of binary mixtures of ionic liquids with bis(trifluoromethylsulfonyl)imide as the common anion. J Chem Thermodyn. 2013;64:22–7.
Gnanakumari P, Venkatesu P, Rama Mohan K, Prabhakara Rao MV, Prasad DHL. Excess volumes and excess enthalpies of N-methyl-2-pyrrolidone with branched alcohols. Fluid Phase Equilib. 2007;252:137–42.
Palm R, Kurig H, Tonurist K, Janes A, Lust E. Is the mixture of 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium tetrafluoroborate applicable as electrolyte in electrical double layer capacitors? Electrochem Commun. 2012;22:203–6.
Chaurasia SK, Singh RK, Chandra S. Structural and transport studies on polymeric membranes of PEO containing ionic liquid, EMIM-TY: evidence of complexation. Solid State Ion. 2011;183:32–9.
Gonzalez EJ, Requejo PF, Dominguez A, Macedo EA. Physical properties of binary alcohol + ionic liquid mixtures at several temperatures and atmospheric pressure. J Solut Chem. 2013;42:746–63.
Balaji R, Sankar MG, Sekhar MC, Shekar MC. Thermophysical and spectroscopic properties of binary liquid systems: acetophenone/cyclopentanone/cyclohexanone with N-methylformamide. Phys Chem Liq. 2016;54:422–39.
Dragoescu D. Refractive indices and their related properties for several binary mixtures containing cyclic ketones and chloroalkanes. J Mol Liq. 2015;209:713–22.
Kumari PG, Venkatesu P, Hofman T, Rao MVP. Excess molar enthalpies and vapor–liquid equilibrium for N-methyl-2-pyrrolidone with ketones. J Chem Eng Data. 2010;55:69–73.
Gowrisankar M, Venkateswarlu P, Siva Kumar K, Sivarambabu S. Volumetric, speed of sound data and viscosity at (303.15 and 308.15) K for the binary mixtures of N,N-dimethylaniline + aliphatic ketones (C3–C5), +4-methyl-2-pentanone, +acetophenone, +cyclicketones. J Ind Eng Chem. 2014;20:405–18.
Andriyko YO, Reischl W, Nauer GE. Trialkyl-substituted imidazolium-based ionic liquids for electrochemical applications: basic physicochemical properties. J Chem Eng Data. 2009;54:855–60.
Ciocirlan O, Iulian O. Properties of pure 1-butyl-2,3-dimethylimidazolium tetrafluoroborate ionic liquid and its binary mixtures with dimethyl sulfoxide and acetonitrile. J Chem Eng Data. 2012;57:3142–8.
Sunkara GR, Tadavarthi MM, Tadekoru VK, Tadikonda SK, Bezawada SR. Density, refractive index, and speed of sound of the binary mixture of 1-butyl-3-methylimidazolium tetrafluoroborate +N-Vinyl-2-pyrrolidinone from T = (298.15 to 323.15) K at atmospheric pressure. J Chem Eng Data. 2015;60:886–94.
Gupta H, Kataria J, Sharma D, Sharma VK. Topological investigations of molecular interactions in binary ionic liquid mixtures with a common ion: excess molar volumes, excess isentropic compressibilities, excess molar enthalpies and excess molar heat capacities. J Chem Thermodyn. 2016;103:189–205.
Gupta H, Solanki S, Sharma VK. Topological analysis of thermodynamic properties of binary mixtures containing 1-butyl-3-methylimidazolium tetrafluoroborate and cycloalkanones. J Therm Anal Calorim. 2017;127:2459–72.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and methods of purification. 4th ed. New York: Wiley; 1986.
Scholz E. Karl Fischer titration. Berlin: Springer; 1984.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Saini N, Yadav JS, Jangra SK, Sharma D, Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toulidine with pyridine and picolines: excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Malham IB, Turmine M. Viscosities and refractive indices of binary mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate and 1-butyl-2,3-dimethylimidazolium tetrafluoroborate with water at 298 K. J Chem Thermodyn. 2008;40:718–23.
Pal A, Kumar B, Kang TS. Effect of structural alteration of ionic liquid on their bulk and molecular level interactions with ethylene glycol. Fluid Phase Equilib. 2013;358:241–9.
Pal A, Kumar B. Volumetric and acoustic properties of binary mixtures of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4] with alkoxyalkanols at different temperatures. J Chem Eng Data. 2012;57:688–95.
Seki S, Tsuzuki S, Hayamizu K, Umebayashi Y, Serizawa N, Takei K, Miyashiro H. Comprehensive refractive index property for room-temperature ionic liquids. J Chem Eng Data. 2012;57:2211–6.
Stoppa A, Zech O, Kunz W, Buchner R. The conductivity of imidazolium-based ionic liquids from (−35 to 195) °C. A. Variation of Cation’s alkyl chain. J Chem Eng Data. 2010;55:1768–73.
Reddy MS, Nayeem SM, Raju KTSS, Babu BH. The study of solute–solvent interactions in 1-ethyl-3-methylimidazolium tetrafluoroborate +2-ethoxyethanol from density, speed of sound, and refractive index measurements. J Therm Anal Calorim. 2016;124:959–71.
Vercher E, Llopis FJ, Gonzalez-Alfaro V, Miguel PJ, Orchilles V, Martinez- Andreu A. Volumetric properties, viscosities and refractive indices of binary liquid mixtures of tetrafluoroborate-based ionic liquids with methanol at several temperatures. J Chem Thermodyn. 2015;90:174–84.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3-methylimidazolium tetrafluoroborate and cycloalkanone mixtures. J Therm Anal Calorim. 2014;118:431–47.
Ciocirlan O, Teodorescu M, Dragoesce D, Iulian O, Barhala A. Densities and excess molar volumes of the binary mixtures of cyclopentanone with chloroalkanes at T = (288.15, 298.15, 308.15, and 318.15) K. J Chem Eng Data. 2010;55:3891–5.
Dragoescu D, Teodorescu M, Barhala A. Isothermal (vapour + liquid) equilibria and excess Gibbs free energies in some binary (cyclopentanone + chloroalkane) mixtures at temperatures from 298.15 K to 318.15 K. J Chem Thermodyn. 2007;39:1452–7.
Bermudez-Salguero C, Gracia-Fadrique J, Calvo E, Amigo A. Densities, refractive indices, speeds of sound, and surface tensions for dilute aqueous solutions of 2-methyl-1-propanol, cyclopentanone, cyclohexanone, cyclohexanol, and ethyl acetoacetate at 298.15 K. J Chem Eng Data. 2011;56:3823–9.
Ciocirlan O, Teodorescu M, Dragoescu D, Iulian O, Barhala A. Densities and excess molar volumes of the binary mixtures of cyclohexanone with chloroalkanes at temperatures between (288.15 and 318.15) K. J Chem Eng Data. 2010;55:968–73.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Sabbah R, Xu-Wu A, Chickos JS, Leitao MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:93–204.
Gupta H, Chandrasekhar M, Krishna TS, Sharma VK. Thermodynamic properties of mixtures containing 1-butyl-2,3-dimethylimidazolium tetrafluoroborate and cyclopentanone or cyclohexanone. J Mol Liq. 2017;231:225–37.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Gupta H, Malik S, Sharma VK. Excess molar volumes and excess isentropic compressibilities of ternary mixtures containing ionic liquids and cyclic alkanone. J Chem Thermodyn. 2017;112:86–102.
Gupta H, Malik S, Chandrasekhar M, Sharma VK. Thermodynamic investigations of excess heat capacities of ternary liquid mixtures containing [Bmmim][BF4] + [Bmim][BF4] or [Emim][BF4] + cyclopentanone or cyclohexanone. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6587-7.
Amigó JM, Gálvez J, Villar VM. A review on molecular topology: applying graph theory to drug discovery and design. Naturwissenschaften. 2009;96:749–61.
Kier LB, Hall LH. Molecular connectivity in chemistry and drug research. New York: Academic Press; 1976.
Hoyosa H. Topological index. A newly proposed quantity characterizing the topological nature of structural isomers of saturated hydrocarbons. Bull Chem Soc Jpn. 1971;44:2332–9.
Balaban AT. Highly discriminating distance-based topological index. Chem Phys Lett. 1982;89:399–404.
Wiener H. Structural determination of paraffin boiling points. J Am Chem Soc. 1947;69:17–20.
Vaz PD, Ribeiro-Claro PJA. Strong experimental evidence of C–H···O hydrogen bonds in cyclopentanone: the splitting of the ν(C=O) mode revisited. J Phys Chem A. 2003;107:6301–5.
Valadbeigi Y, Farrokhpour H, Tabrizchi M. Effect of hydration on the kinetics of proton-bound dimer formation: experimental and theoretical study. J Phys Chem A. 2014;118:7663–71.
Andriyko Y, Andriiko A, Babushkina OB, Nauer GE. Electrochemistry of TiF4 in 1-butyl-2,3-dimethylimidazolium tetrafluoroborate. Electrochim Acta. 2010;55:1081–9.
Rao CNR. Chemical application of infrared spectroscopy. London: Academic Press; 1963.
Swapnil AD, Kailas LW, Mahesh NV, Diwakar ZS, ChangKyoo Y. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab J Chem. 2016;9:578–87.
Silverstein RM, Bassler GC, Morrik TC. Spectroscopic identification of organic compounds. 5th ed. Singapore: Wiley; 1991.
Huggins ML. The thermodynamic properties of liquids, including solutions. I. Intermolecular energies in monotonic liquids and their mixtures. J Phys Chem. 1970;74:371–8.
Huggins ML. The thermodynamic properties of liquids, including solutions: part 2. Polymer solutions considered as ditonic systems. Polymer. 1971;12:389–99.
Singh PP, Bhatia M. Energetics of molecular interactions in binary mixtures of non-electrolytes containing a salt. J Chem Soc Faraday Trans. 1989;1:3807–12.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1983;66:37–73.
Singh PP, Nigam RK, Singh KC, Sharma VK. Topological aspects of the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1981;46:175–90.
Kier LB, Yalkowsky SH, Sinkula AA, Valvani SC. Physico-chemical properties of drugs. New York: Marcel Dekker; 1980. p. 282–95 (Chapter 9).
Acknowledgements
The authors are thankful to Mr. K. Chandrasekhar Reddy, Sri Sai Baba National College, Anantapur, Andhra Pradesh, for providing Gaussian-09 facility and Centre for Development of Advanced Computing (C-DAC), Pune, India, for providing the computational work. V. K. Sharma is grateful to University Grant Commission (UGC), New Delhi, for the award of Special Assistance Programme (SAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, H., Malik, S. & Sharma, V.K. Excess molar enthalpies for [Bmmim][BF4] + [Bmim][BF4] or [Emim][BF4] + cyclopentanone or cyclohexanone mixtures. J Therm Anal Calorim 136, 1383–1394 (2019). https://doi.org/10.1007/s10973-018-7770-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7770-1