Abstract
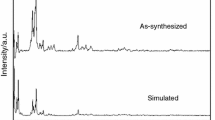
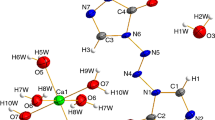
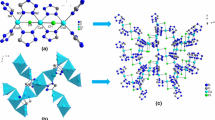
The molar heat capacities of one–three-dimensional metal–organic frameworks Al4(OH)2(OCH3)4(H2N-BDC)3 (CAU-1) were measured by temperature-modulated differential scanning calorimetry (TMDSC) over the temperature range from 213 to 393 K for the first time. No phase transition or thermal anomaly was observed in the experimental temperature range. The fundamental thermodynamic parameters such as entropy and enthalpy relative to 298.15 K were calculated based on the experimentally determined molar heat capacities. The compound was characterized by elemental analysis, powder XRD, FT-IR spectrum. Moreover, the thermal stabilities and decomposition mechanisms of hydrated phase and dehydrated phase of CAU-1 were investigated by thermogravimetric spectrometer in the temperature range 298–1023 K.






Similar content being viewed by others
References
Stock N, Biswas S. Synthesis of metal–organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites. Chem Rev. 2012;112(2):933–69. https://doi.org/10.1021/cr200304e.
Hosny NM. Solvothermal synthesis, thermal and adsorption properties of metal–organic frameworks Zn and CoZn(DPB). J Therm Anal Calorim. 2015;122(1):89–95. https://doi.org/10.1007/s10973-015-4721-y.
Reinsch H. “Green” synthesis of metal–organic frameworks. Eur J Inorg Chem. 2016;2016(27):4290–9. https://doi.org/10.1002/ejic.201600286.
Hosny NM, Al-Hussaini AS, Nowesser N, Zoromba MS. Effect of inclusion of some transition metal ions and use of the doped polymer in synthesizing α-Fe2O3 nanoparticles via thermal decomposition rout. J Therm Anal Calorim. 2016;124(1):287–93. https://doi.org/10.1007/s10973-015-5121-z.
He Y, Zhou W, Qian G, Chen B. Methane storage in metal–organic frameworks. Chem Soc Rev. 2014;43(16):5657–78. https://doi.org/10.1039/C4CS00032C.
Seoane B, Coronas J, Gascon I, Benavides ME, Karvan O, Caro J, et al. Metal–organic framework based mixed matrix membranes: a solution for highly efficient CO2 capture? Chem Soc Rev. 2015;44(8):2421–54. https://doi.org/10.1039/C4CS00437J.
Andirova D, Cogswell CF, Lei Y, Choi S. Effect of the structural constituents of metal organic frameworks on carbon dioxide capture. Microporous Mesoporous Mater. 2016;219:276–305. https://doi.org/10.1016/j.micromeso.2015.07.029.
Lin Y, Kong C, Zhang Q, Chen L. Metal–organic frameworks for carbon dioxide capture and methane storage. Adv Energy Mater. 2017;7(4):1601296. https://doi.org/10.1002/aenm.201601296.
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su C-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem Soc Rev. 2014;43(16):6011–61. https://doi.org/10.1039/C4CS00094C.
Chughtai AH, Ahmad N, Younus HA, Laypkov A, Verpoort F. metal–organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem Soc Rev. 2015;44(19):6804–49. https://doi.org/10.1039/C4CS00395K.
Hu Z, Deibert BJ, Li J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem Soc Rev. 2014;43(16):5815–40. https://doi.org/10.1039/C4CS00010B.
Qiu S, Xue M, Zhu G. metal–organic framework membranes: from synthesis to separation application. Chem Soc Rev. 2014;43(16):6116–40. https://doi.org/10.1039/C4CS00159A.
Banerjee D, Cairns AJ, Liu J, Motkuri RK, Nune SK, Fernandez CA, et al. Potential of metal–organic frameworks for separation of xenon and krypton. Acc Chem Res. 2015;48(2):211–9. https://doi.org/10.1021/ar5003126.
He C, Liu D, Lin W. Nanomedicine applications of hybrid nanomaterials built from metal-ligand coordination bonds: nanoscale metal–organic frameworks and nanoscale coordination polymers. Chem Rev. 2015;115(19):11079–108. https://doi.org/10.1021/acs.chemrev.5b00125.
Wunderlich B. The tribulations and successes on the road from DSC to TMDSC in the 20th century the prospects for the 21st century. J Therm Anal Calorim. 2004;78(1):7–31. https://doi.org/10.1023/b:jtan.0000042150.03836.27.
Song LF, Jiao CL, Jiang CH, Zhang JA, Sun LX, Xu F, et al. Heat capacities and thermodynamic properties of MgNDC. J Therm Anal Calorim. 2011;103(1):365–72. https://doi.org/10.1007/s10973-010-0777-x.
Androsch R. Heat capacity measurements using temperature-modulated heat flux DSC with close control of the heater temperature. J Therm Anal Calorim. 2000;61(1):75–89. https://doi.org/10.1023/a:1010104406353.
Wunderlich B, Jin YM, Boller A. Mathematical description of differential scanning calorimetry based on periodic temperature modulation. Thermochim Acta. 1994;238:277–93. https://doi.org/10.1016/s0040-6031(94)85214-6.
Danley RL. New modulated DSC measurement technique. Thermochim Acta. 2003;402(1–2):91–8.
Wunderlich B. The contributions of MDSC to the understanding of the thermodynamics of polymers. J Therm Anal Calorim. 2006;85(1):179–87. https://doi.org/10.1007/s10973-005-7347-7.
Ahnfeldt T, Guillou N, Gunzelmann D, Margiolaki I, Loiseau T, Ferey G, et al. Al4(OH)2(OCH3)4(H2N-bdc)3·xH2O: a 12-connected porous metal–organic framework with an unprecedented aluminum-containing brick. Angew Chem Int Ed. 2009;48(28):5163–6. https://doi.org/10.1002/anie.200901409.
Schlegel M-C, Tobbens D, Svetogorov R, Kruger M, Stock N, Reinsch H, et al. Conformation-controlled hydrogen storage in the CAU-1 metal–organic framework. PCCP. 2016;18(42):29258–67. https://doi.org/10.1039/c6cp05310f.
Yin H, Wang J, Xie Z, Yang J, Bai J, Lu J, et al. A highly permeable and selective amino-functionalized MOF CAU-1 membrane for CO2–N2 separation. Chem Commun. 2014;50(28):3699–701. https://doi.org/10.1039/c4cc00068d.
Xie L, Liu D, Huang H, Yang Q, Zhong C. Efficient capture of nitrobenzene from waste water using metal–organic frameworks. Chem Eng J. 2014;246:142–9. https://doi.org/10.1016/j.cej.2014.02.070.
Hartmann M, Fischer M. Amino-functionalized basic catalysts with MIL-101 structure. Microporous Mesoporous Mater. 2012;164:38–43. https://doi.org/10.1016/j.micromeso.2012.06.044.
Archer DG. Thermodynamic properties of synthetic sapphire (α-Al2O3), standard reference material 720 and the effect of temperature-scale differences on thermodynamic properties. J Phys Chem Ref Data. 1993;22(6):1441–53.
Ginnings DC, Furukawa GT. Heat capacity standards for the range 14 to 1200 K. J Am Chem Soc. 1953;75(3):522–7. https://doi.org/10.1021/ja01099a004.
Jiang C-H, Song L-F, Jiao C-L, Zhang J, Sun L-X, Xu F, et al. Determination of heat capacities and thermodynamic properties of two structurally unrelated but isotypic calcium and manganese(II) 2,6-naphthalene dicarboxylate-based MOFs. J Therm Anal Calorim. 2011;103(3):1095–103. https://doi.org/10.1007/s10973-010-1197-7.
Song L-F, Jiao C-L, Jiang C-H, Zhang J, Sun L-X, Xu F, et al. Heat capacities and thermodynamic properties of MgNDC. J Therm Anal Calorim. 2011;103(1):365–72. https://doi.org/10.1007/s10973-010-0777-x.
Ferey G, Mellot-Draznieks C, Serre C, Millange F, Dutour J, Surble S, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science. 2005;309(5743):2040–2. https://doi.org/10.1126/science.1116275.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21503129, 61571062, 21572126, 21675109) and Education Department of Henan Province (No. 15A150073).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, S., Sun, LX., Liu, LT. et al. Determination of heat capacities and thermodynamic properties of Al4(OH)2(OCH3)4(H2N-BDC)3. J Therm Anal Calorim 135, 3233–3239 (2019). https://doi.org/10.1007/s10973-018-7608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7608-x