Abstract
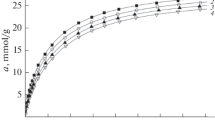
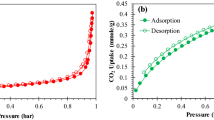
Microporous materials have adsorptive property because of their pore structures. In this paper, the adsorption heat of two boron-containing microporous materials for M2[Ga(B5O10)]·4H2O (M = K, Rb) in some organic solvents was measured at 298.15 K by using microcalorimeter. The results indicate that the larger the pore size, the higher is the heat of adsorption and the higher is the adsorption capacity. In addition, the thermokinetics of adsorption process of K2[Ga(B5O10)]·4H2O in methanol was also studied. Applying thermokinetic model, thermokinetic parameters including rate constants k, reaction order n, activation energy Ea, and pre-exponential factor A for the adsorption process were simultaneously acquired.






Similar content being viewed by others
References
Cheetham A, Ferey K, Loiseau GT. Open-framework inorganic materials. Angew Chem Int Ed. 1999;38:3268–92.
Liu ZH, Yang P, Li P. K2[Ga(B5O10)]·4H2O: the first chiral zeolite-like galloborate with large odd 11-ring channels. Inorg Chem. 2007;46:2965–7.
Li SY, Yang P, Liu ZH. Synthesis, characterization, and thermochemical properties of a microporous crystal material for Rb2[Ga(B5O10)]·4H2O. J Chem Eng Data. 2012;57:1964–9.
Navrotsky A, Trofymluk O, Levchenko AA. Thermochemistry of microporous and mesoporous materials. Chem Rev. 2009;109:3885–902.
Huang PP, Zhao JJ, Liu ZH. Standard molar enthalpies of formation for a series of microporous crystals of Na2[MIIB3P2O11(OH)]·0.67H2O (MII = Mg, Mn, Fe Co, Ni, Cu, Zn). J Chem Thermodyn. 2012;55:213–7.
Lv Y, Li N, Tang M, Liu ZH. Thermodynamic properties of microporous crystals for two hydrated aluminoborates, K2[Al(B5O10)]·4H2O and (NH4)2[Al(B5O10)]·4H2O. J Chem Thermodyn. 2013;58:129–33.
Kang QY, Song Q, Li SY, Liu ZH. Thermodynamic properties of microporous materials for two borophosphates, K[ZnBP2O8] and NH4[ZnBP2O8]. J Chem Thermodyn. 2014;69:43–7.
Liang P, Kang QY, Du L, Liu ZH. Thermochemical properties for a series of microporous borophosphates of MI[ZnBP2O8] (MI = Na, K, Rb, Cs). J Chem Thermodyn. 2014;76:24–8.
Duh YS, Kao CS, William Lee WL. Chemical kinetics on thermal decompositions of di-tert-butyl peroxide studied by calorimetry. J Therm Anal Calorim. 2017;127:1071–87.
Aghili S, Panjepour M, Meratian M. Kinetic analysis of formation of boron trioxide from thermal decomposition of boric acid under non-isothermal conditions. J Therm Anal Calorim. 2018;131:2443–55.
Erceg M, Krešić I, Jakić M, Andričić B. Kinetic analysis of poly(ethylene oxide)/lithium montmorillonite nanocomposites. J Therm Anal Calorim. 2017;127:789–97.
Mateescu M, Budiul M, Albu P, Vlase G, Vlase T. Thermal behavior and kinetic study of degradation for adamantan-2-one versus memantine hydrochloride. J Therm Anal Calorim. 2017;130:391–6.
Huang AC, Chuang YK, Huang CF, Shu CM. Thermokinetic analysis of the stability of malic and salicylic acids in cosmeceutical formulations containing metal oxides. J Therm Anal Calorim. 2018;132:165–72.
Yang HC, Lee SY, Choi YC, Yang IH, Chung DY. Thermokinetic analysis of spent ion-exchange resins for the optimization of carbonization reactor condition. J Therm Anal Calorim. 2017;127:587–95.
Ji M, Liu MY, Gao SL, Shi QZ. A new microcalorimeter for measuring thermal effects. Instrum Sci Technol. 2001;29:53–7.
Li LQ, Song Q, Li P, Huang HS, Liu ZH. Synthesis, characterization, and thermochemical property of a novel mixed alkali metal borate: NaCs[B10O14(OH)4]. J Therm Anal Calorim. 2014;116:1019–25.
Gao YH, Liu ZH. Hydrothermal synthesis and thermodynamic properties of 2ZnO·3B2O3·3H2O. J Chem Thermodyn. 2009;41:775–8.
Gao SL, Chen SP, Hu RZ, Li HY, Shi QZ. Derivation and application of thermodynamic equations. Chin J Inorg Chem. 2002;18:362–6 (in Chinese).
Wang XL, Xia ZQ, Wei W, Xie G, Chen SP, Gao SL. Synthesis, structure, and thermodynamics of a lanthanide coordination compound incorporating 5-nitroisophthalic acid. J Chem Thermodyn. 2012;55:124–9.
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No. 21173143). The authors thank the guidance of Prof. Zhi-Hong Liu, Shaanxi Normal University, P. R. China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, SY., Liang, P. Determination of adsorption heat of two boron-containing microporous materials in some organic solvents by microcalorimetry. J Therm Anal Calorim 134, 2241–2246 (2018). https://doi.org/10.1007/s10973-018-7606-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7606-z