Abstract
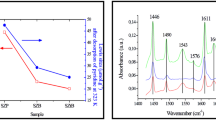
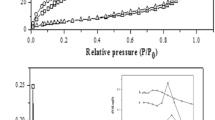
X-ray amorphous precipitated zirconium hydrophosphate ZrP has been subjected to hydrothermal, microwave and mechanochemical modification. Modified samples have been studied using XRD, FTIR spectroscopy, adsorption–desorption of nitrogen, temperature-programmed desorption of ammonia and pyridine adsorption. They possess higher surface area and more developed and accessible pore structure compared with unmodified ZrP. They also have higher concentration of acid sites. The latter are predominantly represented by weak and strong centers. Modified samples also are more active in the process of propan-2-ol dehydration compared with initial ZrP: The temperature of propan-2-ol total conversion reduces from 295 to 193–199 °C. This temperature depends on total pore volume, pore size and total acidity of ZrP samples. Catalysts possessing mesopores size ≥ 4 nm, total pore volume > 0.4 cm3 g−1 and total acidity > 0.4 mmol g−1 exhibit maximal activity. The modified ZrP catalysts are not only efficient but also recyclable since can be used at least three times without decline in the catalytic performances.









Similar content being viewed by others
References
Clearfield A, Costantino U. Comprehensive supramolecular chemistry. Amsterdam: Elsevier; 1996.
Corma A. Solid acid catalysts. Curr Opin Solid State Mater Sci. 1997;2:63–75.
Mendes LC, Silva DF, Araujo LJF, Lino AS. Zirconium phosphate organically intercalated/exfoliated with long chain amine. J Therm Anal Calorim. 2014;118:1461–9.
Bogdanov SG, Valiev EZ, Dorofeev YA, Pirogov AN, Sharygin LM, Moiseev EN, Galkin VM. Structure of zirconium phosphate gels produced by the sol–gel method. J Phys Condens Matter. 1997;9:4031–9.
Khalameida S, Sydorchuk V, Skubiszewska-Zięba J, Charmas B, Skwarek E, Janusz W. Hydrothermal, microwave and mechanochemical modification of amorphous zirconium phosphate structure. J Therm Anal Calorim. 2017;128:795–806.
Tanabe K, Misono M, Hattori H, Ono Y. New solid acids and bases: their catalytic properties. Amsterdam: Elsevier; 1989.
Clearfield A, Thakur DS. Zirconium and titanium phosphates as catalysts: a review. Appl Catal. 1986;26:1–26.
Guerrero-Ruiz A, Rodriguez-Ramos I, Fierro JLG, López AJ, Pastor PO, Torres PM. Catalytic activity of layered α- (tin or zirconium) phosphates and chromia-pillared derivatives for isopropyl alcohol decomposition. Appl Catal A Gen. 1992;92:81–92.
Liu Q, He H, Chao ZS, Xie J, Ruchenstein E. Synthesis of mesoporous chromium phosphates via solid-state reaction at low temperature. New J Chem. 2012;36:139–47.
Perego C, Millini R. Porous materials in catalysis: challenges for mesoporous materials. Chem Soc Rev. 2013;42:3956–76.
Fernandes VJ, Araujo AS, Fernandes GJT. Thermal analysis applied to solid catalysts acidity, activity and regeneration. J Therm Anal Calorim. 1999;56:275–85.
Leofanti G, Padovan M, Tozzola G, Venturelli B. Surface area and pore texture of catalysts. Catal Today. 1998;41:207–19.
Turco M, Ciambelli P, Bagnasco G, Ginestra AL, Galli P, Ferragina C. TPD study of NH3 adsorbed by different phases of zirconium phosphate. J Catal. 1989;117:355–61.
Senchylo EV, Struzhko VL, Solomakha VN. Effect of synthesis conditions on formation of thermally stable mesoporous zirconium phosphate. Theor Exp Chem. 2014;50:257–64.
Cao D, Yu B, Zhang S, Cui L, Zhang J, Cai W. Isosorbide production from sorbitol over porous zirconium phosphatecatalyst. Appl Catal A Gen. 2016;528:59–66.
Niwa M, Katada N. New method for the temperature-programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: a Review. Chem Rec. 2013;13:432–55.
Emeis CA. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J Catal. 1993;141:347–54.
Diyuk VE, Mariychuk RT, Lisnyak VV. Functionalization of activated carbon surface with sulfonated styrene as a facile route for solid acids preparation. Mater Chem Phys. 2016;184:138–45.
Bedia J, Ruiz-Rosas R, Rodríguez-Mirasol J, Cordero T. A kinetic study of 2-propanol dehydration on carbon acid catalysts. J Catal. 2010;271:33–42.
Vidruk R, Landau MV, Herskowitz M, Ezersky V, Goldbourt A. Control of surface acidity and catalytic activity of γ-Al2O3 by adjusting the nanocrystalline contact interface. J Catal. 2011;282:215–27.
Fulvioa PF, Mayesa RT, Bauera JC, Wanga X, Mahurina SM, Veith GM, Dai S. “One-pot” synthesis of phosphorylated mesoporous carbon heterogeneous catalysts with tailored surface acidity. Catal Today. 2012;186:12–9.
Pizzio LR, Cáceres CV, Blanco MN. Acid catalysts prepared by impregnation of tungstophosphoric acid solutions on different supports. Appl Catal A Gen. 1998;167:283–94.
Bedia J, Rosas JM, Vera D, Rodríguez-Mirasol J, Cordero T. Isopropanol decomposition on carbon based acid and basic catalysts. Catal Today. 2010;158:89–96.
Zeng Y, Fan C, Do DD, Nicholson D. Evaporation from an ink-bottle pore: mechanisms of adsorption and desorption. Ind Eng Chem Res. 2014;53:15467–74.
Gheorghiu S, Coppens MO. Optimal bimodal pore networks for heterogeneous catalysis. Am Inst Chem Eng J. 2004;50:812–20.
Choi Y, Yun YS, Park H, Park DS, Yun D, Yi J. A facile approach for the preparation of tunable acid nano-catalysts with a hierarchically mesoporous structure. Chem Commun. 2014;50:7652–5.
Restivo TAG, Mello-Castanho SRH, Tenorio JA. TG/DTA-MS evaluation of methane cracking and coking on doped nickel–zirconia based cermets. J Therm Anal Calorim. 2014;118:75–81.
de Souza G, Marcilio NR, Perez-Lopez OW. Influence of the Ni/Al ratio on Ni–Al mixed oxides and their catalytic properties for ethanol decomposition. J Therm Anal Calorim. 2017;128:735–44.
Ahmed R, Sinnathambi CM, Subbarao D. Kinetics of de-coking of spent reforming catalyst. J Appl Sci. 2011;2011(11):1225–30.
Argyle MD, Bartholomew CH. Heterogeneous catalyst deactivation and regeneration: a review. Catalysts. 2015;5:145–269.
Hansen TW, Delariva AT, Challa SR, Datye AK. Sintering of catalytic nanoparticles: particle migration or ostwald ripening? Acc Chem Res. 2013;46:1720–30.
Slade RCT, Knowles JA, Jones DJ, Rozière J. The isomorphous acid salts α-Zr(HPO4)2 H2O, α-Ti(HPO4)2 H2O and α-Zr(HAsO4)2 H2O. Comparative thermochemistry and vibrational spectroscopy. Solid State Ionics. 1997;96:9–19.
Horsley SE, Nowell DV, Stewart DT. The infrared and Raman spectra of α-zirconium phosphate. Spectrochim Acta. 1974;30A:535–41.
Gu M, Yu D, Zhang H, Sun P, Huang H. Metal (IV) phosphates as solid catalysts for selective dehydration of sorbitol to isosorbide. Catal Lett. 2009;133:214–20.
Nikitina MA, Ivanova II. Conversion of 2,3-butanediol over phosphate catalysts. Chem Cat Chem. 2016;8:1346–53.
Busca G. The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys Chem Chem Phys. 1999;1:723–36.
Jiménez-Jiménez J, Maireles-Torres P, Olivera-Pastor P, Rodríguez-Castellón E, Jiménez-López A, Jones DJ, Rozire J. Surfactant-assisted synthesis of a mesoporous form of zirconium phosphate with acidic properties. Adv Mater. 1998;10:812–5.
Diyuk VE, Zaderko AN, Grishchenko LM, Yatsymyrskiy AV, Lisnyak VV. Efficient carbon-based acid catalysts for the propan-2-ol dehydration. Catal Commun. 2012;27:33–7.
Jiménez-Jiménez J, Maireles-Torres P, Olivera-Pastor P, Rodríguez-Castellón E, Jiménez-López A. Sol-gel synthesis of dodecyltrimethylammonium-expanded zirconium phosphate and its application to the preparation of acidic porous oligomeric gallium(III)-exchanged materials. Langmuir. 1997;13:2857–62.
Thomas JM, Thomas WJ. Principles and practice of heterogeneous catalysis. Hoboken: Wiley; 2015.
Choi Y, Park H, Yun YS, Yi J. Effects of catalyst pore structure and acid properties on the dehydration of glycerol. Chem Sus Chem Commun. 2015;8:974–9.
Shao P, Huang RYM. Polymeric membrane pervaporation. J Membr Sci. 2007;287:162–79.
Van der Bruggen B, Schaep J, Wilms D, Vandecasteele C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J Membr Sci. 1999;156:29–41.
Borjigin T, Sun F, Zhang J, Cai K, Ren H, Zhu G. A microporous metal–organic framework with high stability for GC separation of alcohols from water. Chem Commun. 2012;48:7613–5.
Moeini V, Deilam M. Determination of molecular diameter by PVT. ISRN Phys Chem. 2012;. doi:10.5402/2012/521827.
Rouquerol J, Avnir D, Fairbridge CW, Everett DH, Haynes JH, Pernicone N, Ramsay JDF, Sing KSW, Unger KK. Recommendations for the characterization of porous solids (Technical Report). Pure Appl Chem. 1994;66:1739–58.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalameida, S., Diyuk, V., Zaderko, A. et al. The study of modified zirconium catalysts for selective dehydration of propan-2-ol. J Therm Anal Calorim 131, 2361–2371 (2018). https://doi.org/10.1007/s10973-017-6733-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6733-2