Abstract
Linear bio-based polyester polyols were prepared with the use of succinic acid and 1.3-propanediol (both with natural origin). As a catalyst was used tetraisopropyl orthotitanate (TPT). In order to determine the effect of various catalyst content on the thermal degradation characteristics, three different TPT amounts, as a 1.3-propanediol equivalent, were used, namely 0.1 mass% (PPS-0.1), 0.2 mass% (PPS-0.2) and 0.25 mass% (PPS-0.25). The reference polyol was prepared without catalyst employment (PPS-0.0). Fourier transform infrared spectroscopy was used to confirm molecular structure of the resulted polyols. The structure was also corroborated by 1H NMR measurements, what confirmed nonsignificant catalyst amount impact on the structure of the prepared polyester polyols. Differential scanning calorimetry was carried out for glass transition temperature and melting point determination. The thermogravimetric analysis allowed to observe high thermal stability both under inert and oxidative atmosphere. This analysis affirmed also that the catalyst content did not influence significantly on the thermal degradation characteristics of the prepared polyols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyols constitute one of the major components for the polyurethanes synthesis [1]. They are usually liquid, reactive substances mostly terminated by the hydroxyl or partially amine groups [2], which are responsible for the reaction with isocyanates [3, 4]. Polyester polyols represent the second most important group (beside polyether polyols) with around 18% of the polyols global usage [5]. The polyurethane materials obtained with the use of polyester polyols are less resistant to hydrolysis compared to the polyether polyols. However, it makes them more favourable due to the biodegradability [6–8]. Polyurethanes based on the polyester polyols have better thermal and fire resistance than the polyether-based PUR and superior solvent resistance. The greatest value of polyester polyols application is the polyurethane elastomers (ca. 43%), flexible foams (ca. 15–18%), adhesives, coating, etc. Furthermore, polyesters give major possibilities to the bio-renewable PUR material obtaining [5].
Currently, readily accessible are the bio-components which allow producing polyester polyols in 100% consisting from bio-resources [9]. One of the most important bio-based monomers for the polyester polyols synthesis is the succinic acid (SA). Commercially available are also glycols, which constitute second monomer taking part in the polycondensation reaction during polyester polyols preparation. Bio-based glycol with the highest global usage, the first widely available in the industry, represents such bio-based glycol as 1.3-propanediol (PDO) (Susterra, DuPont). Currently, these compounds are obtaining by the biotechnological processes through the corn fermentation by such microorganisms as a fungi, yeasts or bacteria [10–13] or fermentation process based on the glucose, sucrose, dextrose and biomass sugars [14, 15], respectively. Other bio-based glycols commonly available constitute also bio-based 1.4-butanediol (bio-BDO) and ethylene glycol (bio-EG). It is well known that the petrochemical-based components have more impurities, which consisted of the different chemical compounds. The different contaminants can lead to the other reaction mechanisms during the polymer synthesis; therefore, it is necessary to find the contaminants impact on the polyester polyols synthesis pathways.
The most important information about polyesters is their thermal stability and melting behaviour. These characteristics give information about the promising behaviour of the polyester during processing. Papageorgiou and Bikiaris [16] carried out the comparative study about crystallisation and melting behaviour of three polyesters based on the succinic acid, namely poly(ethylene succinate), PES, poly(propylene succinate), PPS and poly(butylene succinate), PBS. All measured polymers revealed the same molecular weight. They indicated that the slowest crystallisation rate and the lowest crystallinity degree, among these polyesters, exhibited poly(propylene succinate). The results of the differential scanning calorimetry allowed to find the equilibrium melting points of PES, PPS and PBS at 114, 58 and 133.5 °C. A lower melting point confirmed superior promising behaviour during industrial processes for poly(propylene succinate), where the higher melting points for PES and PBS limited their practical application. Another thermal property with high impact on the polyesters practical employment is glass transition temperature. Bikiaris et al. [17] indicated that the highest glass transition temperature exhibited PES at ca. −11 °C, where the PPS and PBS exhibited this temperature at ca. −35 and −44 °C, respectively. Similar results have been obtained also by the Qiu et al. [18] and Liu et al. [19]. Chrissafis et al. [20] measured thermal decomposition temperature of the poly(propylene succinate) by thermogravimetric measurements. In the results, PPS revealed a very high thermal stability, which confirmed the thermal decomposition temperature at 404 °C. The same researchers indicated that PES and PBS decomposed at 413 and 399 °C, respectively [21]. This temperature can be compared to the degradation temperatures of the aromatic polyesters, and they are higher than thermal decomposition temperatures of other aliphatic polyesters.
In the present work, the synthesis of series of the linear bio-based aliphatic polyester polyols is described. The syntheses were designed to obtain the polyesters with proposed number average molecular weight, which means ca. 2000 g mol−1, and functionality, f = 2. The polycondensation catalyst—tetraisopropyl orthotitanate (TPT), was used in three different amounts. The obtained poly(propylene succinate)s were characterised by structure analysis with the use of Fourier transform infrared spectroscopy and proton nuclear magnetic resonance. Thermal degradation characteristic was determined with the use of thermogravimetric analysis and differential scanning calorimetry.
Measurements
Materials
Bio-based succinic acid (SA) (solid, molecular weight 118.09 g mol−1, purity 98–100%, relative density at 20 °C 0.900 g cm−3) used in this study was obtained from BioAmber Sarnia Inc. (Ontario, Canada). Susterra Propanediol (1.3-propanediol) (liquid, molecular weight 76.09 g mol−1, purity 99.98%, water content by Karl Fischer 12.1 ppm, relative density at 20 °C 1.053 g cm−3, dynamic viscosity at 20 °C 52 mPas) was obtained from DuPont Tate and Lyle Corporation Bio Products (Loudon, Tennessee, USA). Tetraisopropyl orthotitanate, Ti(O-i-Pr)4 (TPT) (liquid, molecular weight 284.22 g mol−1, purity 97%) was used as a catalyst with four different amounts. The catalyst was purchased from TCI Chemicals (India). All other materials and solvents used for the analytical measurement methods for prepared bio-based polyester polyols characterisation were of analytical grade.
Bio-based polyesters synthesis
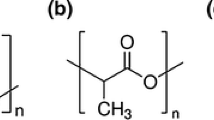
Aliphatic bio-based polyester polyols were prepared with the use of dicarboxylic acid, which was succinic acid, and glycol, which was 1.3-propanediol. Both components used were of a natural origin. Tetraisopropyl orthotitanate (TPT) was used as a catalyst and added in three different amounts, namely 0.1 mass% (PPS-0.1), 0.2 mass% (PPS-0.2) and 0.25 mass% (PPS-0.25). Poly(propylene succinate) was also prepared without catalyst employment (PPS-0.0). All linear bio-based polyester polyols were synthesised in the bulk by two-step polycondensation method (esterification and polycondensation). Figure 1 shows the reaction scheme. The first step was represented by the esterification reaction between a succinic acid (SA) and 1.3-propanediol (PDO). Glycol was always used with an excess. The molar ratio SA/PDO amounted to 1:1.2 which was determined considering the final molar mass expected after full polycondensation (approximately number average molecular weight Mn = 2000 g mol−1 and functionality f = 2). Both of the steps were carried out in the glass reactor, which consisted of a three-neck flask equipped with nitrogen/vacuum inlet, mechanical stirrer, thermometer and condenser. The glass reactor was placed into a heating mantle. The first step of the reaction was carried out under a nitrogen atmosphere. Succinic acid (169 g, 1.43 mol) and 1.3-propanediol (131 g, 1.72 mol) mixture was stirring at 140 °C and kept for 10 h at this temperature (application for patent in the Polish Patent Office, No. P.418808). After the water distillation, the second step, which was the main polycondensation reaction, was carried out. The nitrogen was stopped, the appropriate amount of catalyst was added to reaction mixture, and the temperature was increased up to 160 °C under reduced pressure. During polycondensation, the acidic number was measured. After achieving the value of the acidic number ca. or preferably below 1 mg KOH g−1, the polycondensation was finished.
Polymer characterisation
Acid and hydroxyl number
Carboxyl end group value measurements were taken in accordance with the Polish Standard PN-86/C45051. Samples about 1 g of the prepared polyesters were dissolved in ca. 30 cm3 of acetone at room temperature. Thereafter, the solutions were titrated with the use of a standard solution of potassium hydroxide KOH in distilled water (0.1 mol dm−3) and phenolphthalein as indicator.
Hydroxyl group determination was performed with the use of sample about ca. 0.5 g of polyester. The sample was dissolved in 5 cm3 of the acetic anhydride solution prepared in accordance with the Polish Standard PN-88/C-89082. The solution was refluxed for 30 min. After that, 1 cm3 of pyridine was added and heating for 10 min. Thereafter, 50 cm3 of distilled water was added, and the mixture was cooled to room temperature and titrated with the use of a standard solution of potassium hydroxide KOH in distilled water (0.5 mol dm−3) and phenolphthalein as indicator.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy was used to obtain the spectra of the samples of pure components used in this study (1.3-propanediol and succinic acid) and prepared, without catalyst employment, bio-based polyester polyol (PPS-0). The measurements were taken using a Nicolet 8700 FTIR spectrometer (Thermo Electron Corporation, USA) with the use of ATR technique. The resolution was 4 cm−1. Sixty-four scans in the wavenumber range from 4500 to 500 cm−1 were taken.
Nuclear magnetic resonance (1H NMR)
Proton nuclear magnetic resonance (1H NMR) spectra of the prepared bio-based polyester polyols were obtained with the use of Bruker spectrometer. Operating frequency was 400 MHz for protons. The ca. 10% w v−1 solutions of the poly(propylene succinate) polyesters were prepared in a CDCl3 solvent at ambient temperature. The simulation and iteration of spectra were carried out using Bruker software.
Differential scanning calorimetry
The glass transition temperature and melting point determination of the prepared bio-based polyester polyols were characterised by using a DSC 204 F1 Phoenix Analyzer, equipped with a cooling system. In the first cycle, the samples were heated at a rate of 20 °C min−1 where the temperature ranged from −80 to 100 °C for erasing of the polyols thermal history. Then, they were cooled down to −80 °C at a cooling rate of 10 °C min−1. In the third cycle, the samples were heated up to 100 °C at a rate of 5 °C min−1. All the measurements were taken under nitrogen atmosphere. DSC curves for linear bio-based polyester polyols prepared without catalyst employment and with the highest amount of the catalyst were plotted as the results of the DSC analysis.
Thermogravimetric analysis
The thermogravimetric analysis allowed to characterise the thermal stability of the prepared bio-based polyester polyols. The measurements were taken with the use of an NETZSCH TG 209F3 analyser (Netzsch, Germany). The specimens, weighing in the range from ca. 6–8 mg each, were analysed under an inert (nitrogen) and oxidative (air) atmosphere. The used temperature ranged from 35 to 600 °C at a heating rate of 20 °C min−1. Thermogravimetric (TG) and differential thermogravimetric (DTG) curves for prepared linear bio-based polyester polyols were plotted as the results of the TG analysis.
Results and discussion
Synthesis and characterisation of the obtained poly(propylene succinate)
All prepared polyester polyols were synthesised with the use of well-known two-step polycondensation method. The first step was the esterification reaction, which was conducted for 10 h for all of the prepared polyester polyols without using catalyst. After minimum 60% of water removal, the catalyst was added. The second step, which was the main polycondensation reaction, was carried out by individual time for all synthesised polyesters until achievement of the acid number ca. or preferably lower than 1 mg KOH g−1. Justification of the end point of the polycondensation reaction choice was the carboxyl end group value due to the acid number occurring in some synthetic polyester polyols commonly used in the polyurethane industry. Table 1 shows properties of the prepared polyols.
The addition of the catalyst influenced the time of the second step of the reaction. Bio-based polyester polyol prepared without tetraisopropyl orthotitanate employment exhibited the longest reaction time. For the acid number obtainment at the lowest level, the reaction time totalled 180 h. Increasing catalyst employment resulted in decrease in the polycondensation time. Poly(propylene succinate) prepared with the use of the highest catalyst amount (0.25 mass% as the glycol equivalent) revealed the lowest time for achieving the acid number value at 0.83 mg KOH g−1. The lowest hydroxyl number disclosed the PPS-0.0 sample in 44.60 mg KOH g−1 where the highest value exhibited PPS-0.2 (72.40 mg KOH g−1). All measured hydroxyl number values are similar with the commercially available synthetic polyols used in the polyurethane syntheses.
Chemical structure analysis
The structure analysis was performed using the FTIR and 1H NMR measurements. The Fourier transform infrared spectra of the poly(propylene succinate), prepared without catalyst used, and pure component used for polyesters synthesis (1.3-propanediol and succinic acid) are shown in Fig. 2. The wide peak characteristic for the 1.3-propanediol spectrum in the wavelength range between 3570 and 3170 cm−1 was attributable to the stretching vibrations of hydrogen-bonded hydroxyl groups. For the succinic acid spectrum, the peak assigned to the hydrogen-bonded carboxyl groups stretching vibration appeared as the broad peak centred at 3300–2500 cm−1. The peaks at 3000–2850 cm−1 were assigned to the methylene groups which are visible for glycol and polyester spectra. The methylene group stretching vibration from succinic acid is shifted to wavenumber at 2500–2250 cm−1. Two intensive peaks visible for poly(propylene succinate) spectrum at 1725 and 1150 cm−1 indicated ester groups [22]. The peak at 1725 cm−1 is assigned to carbonyl group stretching vibration related to the ester groups from poly(propylene succinate) [23]. The absorption at 1690 cm−1 visible on the succinic acid spectrum also indicated the –C=O stretching vibration but assigned to the carboxyl group. The bond in the wavenumber at 1150 cm−1 is assigned to C–O–C stretching vibration and at the 1030 cm−1 indicated C–C–O stretching vibration from homopolyester [24]. The peaks at 1170 cm−1 on the glycol spectrum and at 1200 cm−1 on the succinic acid spectrum were also attributed to the stretching C–C–O group vibration but originating from hydroxyl and carboxyl groups, respectively [23].
1H NMR spectra were used to study the structure of the synthesised polyesters (Fig. 3). Based on the spectrum of the poly(propylene succinate) prepared with the 0.2 mass% catalyst employment, the chemical shift of the protons was investigated. The characteristic intensive single peak at 2.63 ppm attributed to methylene protons ‘a’ from succinic acid (–CH2–C(O)–) [17, 25]. Peaks named ‘b’ (–CH2–O–) and ‘c’ (–CH2–) at 4.20 and 2.00 ppm, respectively, attributed to triple and multiple peaks corresponding to methylene protons from propylene glycol (1.3-propanediol) [20, 21]. The intensive single peak at 7.26 ppm attributed to used solvent, which was CDCl3 and the little single peak at 1.56 ppm attributed to the water content in the sample [22, 26, 27]. At the PPS-0.2 sample spectrum, other peaks are also visible in lower intensity which can indicate the end groups of oligomers. The little triple peak at 3.65 ppm named ‘x’ attributed to methylene protons from hydroxyl terminated ends (–CH2–OH) of polyester macromolecules [28]. The peak at 4.35 ppm named ‘z’ attributed to the triple peak corresponding to methylene protons (–CH2–O–) from glycol terminated ends group. Peak named ‘y’ at ca. 1.90 ppm attributed to methylene protons also from glycol terminated end group (–CH2–). Figure 4 illustrates differences between polyesters prepared with the use of a catalyst and without catalyst content. It can be seen that PPS-0.2 sample spectrum indicates the little shift at the 1.90, 3.68 and 4.35 ppm when the PPS-0.0 indicates this peak with lower intensity. Chrissafis et al. [20] explained that these peak intensities can correspond to the lower molecular weight of the synthesised polyester polyols.
Differential scanning calorimetry
The polyols thermal properties are related to their structure and molecular weight. The polyols, which the methyl groups hanging from the main chain resulting in a greater free volume reveal lower glass transition temperature. Moreover, the increasing molecular weight of polyol increases the degree of crystallisation. In consequence, the mobility of the amorphous chains would be restricted by neighbouring crystals. In the sequel, the glass transition temperature will be lower and the melting point will be higher [29, 30]. Figure 5 shows the results of the differential scanning calorimetry measurements of two of the prepared bio-based polyester polyols. The graph presented the curve courses of the poly(propylene succinate)s prepared without catalyst employment (PP-0.0) and with the highest amount of the catalyst (PPS-0.25). The first run was carried out with the heating rate of 20 °C min−1, and the second run was carried out with the heating rate of 5 °C min−1 after quenching under a nitrogen atmosphere with the rate of 10 °C min−1. The addition of the catalyst influenced the melting point and glass transition temperatures of the synthesised bio-based polyester polyols. The catalyst impact on these properties is also related to the effect on the polyol molecular weight. PPS-0.0 sample reached the melting point at 51.5 °C, where the polyol PPS-0.25 revealed this temperature at 47.5 °C. The same tendency is also visible in the case of the glass transition temperature. With the catalyst employment, this temperature decreases from −26.8 to −30.0 °C for PPS-0.0 and PPS-0.25, respectively. The erasing of the polyols thermal history allowed to observe significant changes in the curve courses during the second run. The glass transition temperatures were shifted from −26.8 to −32.3 °C for PPS-0.0 and from −30.0 to −34.5 °C for PPS-0.25. It can be described due to the chain flexibility enhancement after bio-based polyester polyols thermal history erasing. The results indicated also that this history erasing affected the prepared polyols crystallisation. It can be corroborated by the lack of the crystallisation peak at the cooling curves and melting point at the second run curves. Tsai et al. [31] concluded that the odd number of the carbon atoms in the copolyesters backbone relevantly inhibits their crystallisation rate. After thermal history erasing, the prepared bio-based poly(propylene succinate) polyols revealed the lack of the melting point. The same results have been obtained by Papageorgiou and Bikiaris [16]. Moreover, the researchers indicate that the melting temperatures of the semi-crystalline PPS polyesters depended on the thermal history of the samples and it is found to range from 42 to 50 °C or even more. After melt quenching, the poly(propylene succinate) can adopt totally amorphous structure [20].
Thermogravimetric analysis
Thermogravimetric measurements allowed to obtain the thermal decomposition characteristics all of the prepared bio-based polyester polyols. The resulting curves were plotted on the differential thermogravimetric (DTG; Figs. 6, 7) and thermogravimetric (TG; Figs. 8, 9) graphs at heating rate 20 °C min−1 under nitrogen and air atmosphere. The thermal decomposition characteristics are summarised in Table 2. The obtained results allow confirming that oxidative atmosphere influences the thermal degradation temperatures on slightly lower values than inert atmosphere. The similar results were obtained also by Wang et al. [32] and Ciecierska et al. [33].
From the DTG curve courses, it can be seen that increasing catalyst employment during PPS syntheses caused an increase in the speed of the mass loss in the case of inert atmosphere. The highest speed of mass loss revealed polyol prepared with the use of 0.25 mass% of the catalyst. Differences in the DTG curves intensity are more visible in the case of measurements taken under oxidative atmosphere. The catalyst usage caused the increase at the speed of mass loss, but with increasing catalyst amount, the speed falls (in air). The specimen PPS-0.25 revealed the most similar value to reference sample—the lowest speed of mass loss in comparison with other catalysed specimens.
Table 2 shows that the prepared bio-based polyester polyols samples revealed the similarity at the thermal decomposition temperatures in the case of oxidative and inert atmosphere. In Figs. 6 and 7, the small peaks are visible for all samples at the temperature ca. 360 °C. Chrissafis et al. [20] indicated that these little peaks are due to oligomer degradation. For PPS-0.0 and PPS-0.1, peaks at 391 and 395 °C (Fig. 6), respectively, are correlated with the decomposition temperature of the polyesters that exhibited some smaller molecular chain. These polyesters revealed higher polydispersity, and molecules with lower molecular weight decomposed at a lower temperature. The highest temperature of the maximum speed of mass loss under the nitrogen revealed PPS-0.2, where for PPS-0.25, this temperature significantly decreased (407.7 and 405.1, respectively). For measurements prepared in the air, the highest temperature of the maximum speed of mass loss revealed reference sample and then PPS-0.1 and PPS-0.25 (406.5, 400.1 and 399.3, respectively).
Figures 8 and 9 show the TG curve courses where the slight differences are visible. Samples PPS-0.1 and PPS-0.25 revealed faster mass loss at the temperature range from 100 to 360 °C than sample PPS-0.2. In the term of oxidative atmosphere, the PPS-0.1 decomposed first, which is correlated with the data in Table 2. After achieving the temperature 360 °C, the curve courses in Figs. 8 and 9 were intensively dropped which indicated quick decomposition. As can be seen for all measured bio-based polyester polyols after achieving ca. 450 °C, no char residues were found for all samples and both atmospheres. Chrissafis et al. [20] also indicated that the PPS decomposed at the almost whole mass (about 99 mass%).
Conclusions
The series of the linear bio-based polyester polyols were synthesised with planned molecular structure and functionality. The chosen properties were created in accordance with the requirements of the thermoplastic polyurethane industries. The Fourier transform infrared spectra confirmed the ester bonds formation, which are visible at the most intensive peaks at 1725 and 1150 cm−1 on the poly(propylene succinate)s spectra. Also, 1H NMR analysis corroborated the linear polyester polyols obtaining. Increasing catalyst amount influenced the reaction time reduction. Catalyst employment resulted in decrease in the total reaction time from ca. 180 h, for PPS-0.0, to ca. 19 h, for PPS-0.25, respectively. Differential scanning calorimetry results confirmed the impact of the catalyst usage on the melting point and glass transition temperature of the synthesised bio-based polyester polyols. Catalyst employment decreased the melting point from 51.5 °C for PPS-0.0 to 47.5 °C for PPS-0.25. Furthermore, the glass transition temperatures also decreased with the catalyst employment, exactly from −32.3 °C, for PPS-0.0, to −34.5 °C, for PPS-0.25 (at the second heating rate, after the thermal history erasing). The differential thermogravimetric analysis showed that the speed of mass loss of the prepared bio-based polyester polyols increases with the growing catalyst amount. The results indicated that the growing catalyst amount led to polyols, which exibited the shift of the onset decomposition temperature (T5%) from 361.5 to 297.4 °C, under the nitrogen and from 351.5 to 300.0 °C, under oxidative atmosphere, for PPS-0.0 and PPS-0.25, respectively. Nevertheless, the other temperatures confirmed nonsignificant differences in the thermal decomposition characteristics in the case of all samples measured in the both gasses.
References
Rogulska M, Kultys A, Pikus S. The effect of chain extender structure on the properties of new thermoplastic poly(carbonate-urethane)s derived from MDI. J Therm Anal Calorim. 2017;127:2325–39.
Prociak A, Rokicki G, Ryszkowska J. Materiały poliuretanowe. Warszawa: Wydawnictwo Naukowe PWN; 2014.
Szycher M. Handbook of polyurethanes. 2nd ed. Boca Raton: CRC Press; 1999.
Kucinska-Lipka J, Gubanska I, Sienkiewicz M. Thermal and mechanical properties of polyurethanes modified with L-ascorbic acid. J Therm Anal Calorim. 2017;127:1631–8.
Ionescu M. Chemistry and technology of polyols for polyurethane. 1st ed. Billingham: Rapra Technology Limited; 2005.
Król P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog Mater Sci. 2007;52:915–1015.
Kim YD, Kim SC. Effect of chemical structure on the biodegradation of polyurethanes under composting conditions. Polym Degrad Stab. 1998;62:343–52.
Rutkowska M, Krasowska K, Heimowska A, Steinka I, Janik H. Degradation of polyurethanes in sea water. Polym Degrad Stab. 2002;76:233–9.
Miller R. Evaluating the properties and performance of susterra® 1, 3 propanediol and biosuccinium™ sustainable succinic acid in TPU applications. In: CPI polyurethanes 2012 tech. conf. (Internet). 2012. p. 1–19. http://www.reverdia.com/wp-content/uploads/Article-PUMI-Biosuccinium_and_Susterra_03-2013.pdf.
Delhomme C, Weuster-Botz D, Kühn FE. Succinic acid from renewable resources as a C4 building-block chemical—a review of the catalytic possibilities in aqueous media. Green Chem. 2009;11:13.
Bechthold I, Bretz K, Kabasci S, Kopitzky R, Springer A. Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem Eng Technol. 2008;31:647–54.
Kamzolova SV, Yusupova AI, Dedyukhina EG, Chistyakova TI, Kozyreva TM, Morgunov IG. Succinic acid synthesis by ethanol-grown yeasts. Food Technol Biotechnol. 2009;47:144–52.
Nghiem N, Davison B, Suttle B, Richardson G. Production of succinic acid by Anaerobiospirillum succiniciproducens. Appl Biochem Biotechnol. 1997;63(65):565–76.
Kaur G, Srivastava AK, Chand S. Advances in biotechnological production of 1,3-propanediol. Biochem Eng J. 2012;64:106–18.
Garikipati, Svb J, Japs M, Sonico I. WO2014152665A1. WO2014152665A1; 2014.
Papageorgiou GZ, Bikiaris DN. Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study. Polymer. 2005;46:12081–92.
Bikiaris DN, Papageorgiou GZ, Achilias DS. Synthesis and comparative biodegradability studies of three poly(alkylene succinate)s. Polym Degrad Stab. 2006;91:31–43.
Qiu Z, Komura M, Ikehara T, Nishi T. DSC and TMDSC study of melting behaviour of poly(butylene succinate) and poly(ethylene succinate). Polymer. 2003;44:7781–5.
Liu Y, Ranucci E, Lindblad MS, Albertsson AC. New biodegradable polymers from renewable sources—segmented copolyesters of poly(1,3-propanediol succinate) and poly(ethylene glycol). J Bioact Compat Polym. 2002;17:209–19.
Chrissafis K, Paraskevopoulos KM, Bikiaris DN. Thermal degradation kinetics of the biodegradable aliphatic polyester, poly(propylene succinate). Polym Degrad Stab. 2006;91:60–8.
Chrissafis K, Paraskevopoulos KM, Bikiaris DN. Thermal degradation mechanism of poly(ethylene succinate) and poly(butylene succinate): comparative study. Thermochim Acta. 2005;435:142–50.
Zheng L, Li C, Zhang D, Guan G, Xiao Y, Wang D. Multiblock copolymers composed of poly (butylene succinate) and poly (1,2-propylene succinate): effect of molar ratio of diisocyanate to polyester-diols on crosslink densities, thermal properties, mechanical properties and biodegradability. Polym Degrad Stab. 2010;95:1743–50.
Umare SS, Chandure AS, Pandey RA. Synthesis, characterization and biodegradable studies of 1,3-propanediol based polyesters. Polym Degrad Stab. 2007;92:464–79.
Ma X, Chang PR, Yu J, Wang N. Preparation and properties of biodegradable poly (propylene carbonate)/thermoplastic dried starch composites. Carbohydr Polym. 2008;71:229–34.
Bikiaris DN, Papageorgiou GZ, Giliopoulos DJ, Stergiou CA. Correlation between chemical and solid-state structures and enzymatic hydrolysis in novel biodegradable polyesters. The case of poly (propylene alkanedicarboxylate) s. Macromol Biosci. 2008;8:728–40.
Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62:7512–5.
Fulmer GR, Miller AJM, Sherden NH, Gottlieb HE, Nudelman A, Stoltz BM, et al. NMR chemical shifts of trace impurities: common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics. 2010;29:2176–9.
Zheng L, Li C, Huang W, Huang X. Synthesis of high-impact biodegradable multiblock copolymers comprising of poly (butylene succinate) and hexamethylene diisocyanate as chain extender. Polym Adv Technol. 2011;22:279–85.
Saralegi A, Rueda L, Fernández-Darlas B, Mondragon I, Eceiza A, Corcuera MA. Thermoplastic polyurethanes from renewable resources: effect of soft segment chemical structure and molecular weight on morphology and final properties. Polym Int. 2013;62:106–15.
Korley LSTJ, Pate BD, Thomas EL, Hammond PT. Effect of the degree of soft and hard segment ordering on the morphology and mechanical behavior of semicrystalline segmented polyurethanes. Polymer. 2006;47:3073–82.
Tsai CJ, Chang WC, Chen CH, Lu HY, Chen M. Synthesis and characterization of polyesters derived from succinic acid, ethylene glycol and 1,3-propanediol. Eur Polym J. 2008;44:2339–47.
Wang Y, Zhang L, Yang Y, Cai X. The investigation of flammability, thermal stability, heat resistance and mechanical properties of unsaturated polyester resin using AlPi as flame retardant. J Therm Anal Calorim. 2015;122:1331–9.
Ciecierska E, Jurczyk-Kowalska M, Bazarnik P, Kowalski M, Krauze S, Lewandowska M. The influence of carbon fillers on the thermal properties of polyurethane foam. J Therm Anal Calorim. 2016;123:283–91.
Acknowledgements
The authors gratefully acknowledge BioAmber Sarnia Inc. (Canadian corporation) for giving the samples of succinic acid used in this study. The sincere acknowledgements are also directed to the DuPont Tate&Lyle Corporation for supplying the glycol (1.3-propanediol) samples used in this study. Thanks are also due to Mr Dr Michał Strankowski and Mr Dr Łukasz Piszczyk from the Department of Polymers Technology in the Gdansk University of Technology for the differential scanning calorimetry and thermogravimetric measurements preparation of the bio-based polyester polyols described in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Parcheta, P., Datta, J. Structure analysis and thermal degradation characteristics of bio-based poly(propylene succinate)s obtained by using different catalyst amounts. J Therm Anal Calorim 130, 197–206 (2017). https://doi.org/10.1007/s10973-017-6376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6376-3