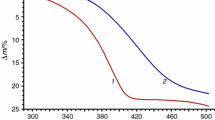
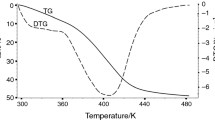
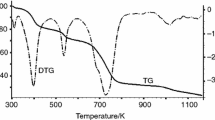
Abstract
Metal–organic frameworks (MOFs) have promising practical applications in gas storage, separation and purification, and catalysis. The standard process for MOF production begins with the synthesis of the inclusion compound. The molecules of the organic solvent used are caught in the channels and caves of the MOF structure. These primary inclusion guest molecules are excluded further by the evacuation or by the heating. We investigate a series of inclusion compounds and study the correlation between their thermal (kinetic) stability and the framework and guest molecule properties. Thermogravimetric curves are used for the kinetic studies. Kinetic parameters of decomposition are estimated within the approaches of non-isothermal kinetics (“model-free” kinetics and nonlinear regression methods). We discuss guest molecular kinetic diameters, guest molecule sizes, shape and polarity, the guest phase state (fluid or solid) within the framework pores, the flexibility of the framework structures, the enthalpy and entropy contributions to the kinetic stability and the inclusion compound properties with very similar frameworks (channel walls with similar chemical constitutions), but with different channels length.






Similar content being viewed by others
References
Guillerm V, Kim D, Eubank JF, Luebke R, Liu X, Adil K, Lah MS, Eddaoudi M. A supermolecular building approach for the design and construction of metal–organic frameworks. Chem Soc Rev. 2014;43:6141–72. doi:10.1039/C4CS00135.
Farha OK, Hupp JT. Rational design, synthesis, purification, and activation of metal–organic framework materials. Acc Chem Res. 2010;43:1166–75. doi:10.1021/ar1000617.
Zhang Z, Zhao Y, Gong O, Li Z, Li J. MOFs for CO2 capture and separation from flue gas mixtures: the effect of multifunctional sites on their adsorption capacity and selectivity. Chem Commun. 2013;49:653–61. doi:10.1039/C2CC35561B.
Bae Y-S, Snurr RQ. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew Chem Int Ed. 2011;50:11586–96. doi:10.1002/anie.201101891.
Tranchemontagne DJ, Park KS, Furukawa H, Eckert J, Knobler CB, Yaghi OM. Hydrogen storage in new metal–organic frameworks. J Phys Chem C. 2012;116:13143–51. doi:10.1021/jp302356q.
Langmi HW, Ren J, North B, Mathe M, Bessarabov D. Hydrogen storage in metal–organic frameworks: a review. Electrochim Acta. 2014;128:368–92. doi:10.1016/j.electacta.2013.10.190.
Yu Y, Ren Y, Shen W, Deng H, Gao Z. Applications of metal–organic frameworks as stationary phases in chromatography. TrAC (Trends Anal Chem). 2013;50:33–41. doi:10.1016/j.trac.2013.04.014.
Van de Voorde B, Bueken B, Denayer J, De Vos D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem Soc Rev. 2014;43:5766–88. doi:10.1039/C4CS00006D.
Liu J, Chen L, Cui H, Zhang J, Zhang L, Su C-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem Soc Rev. 2014;43:6011–61. doi:10.1039/C4CS00094C.
Liu Y, Xuan W, Cui Y. Engineering homochiral metal–organic frameworks for heterogeneous asymmetric catalysis and enantioselective separation. Adv Mat. 2010;22:4112–35. doi:10.1002/adma.201000197.
Hu Z, Deibert BJ, Li J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem Soc Rev. 2014;43:5815–40. doi:10.1039/C4CS00010B.
Pramanik S, Hu Z, Zhang X, Zheng C, Kelly S, Li J. A systematic study of fluorescence-based detection of nitroexplosives and other aromatics in the vapor phase by microporous metal–organic frameworks. Chem A Eur J. 2013;19:15964–71. doi:10.1002/chem.201301194.
Desai AW, Manna B, Karmakar A, Sahu A, Ghosh SK. A water-stable cationic metal–organic framework as a dual adsorbent of oxoanion pollutants. Angew Chem Int Ed. 2016;55:7811–5. doi:10.1002/anie.201600185.
Cunha D, Yahia MB, Hall S, Miller SR, Chevreau H, Elkaïm E, Maurin G, Horcajada P, Serre C. Rationale of drug encapsulation and release from biocompatible porous metal–organic frameworks. Chem Mater. 2013;25:2767–76. doi:10.1021/cm400798p.
Sun C-Y, Qin C, Wang C-G, Su Z-M, Wang S, Wang X-L, Yang G-S, Shao K-Z, Lan Y-Q, Wang E-B. Chiral nanoporous metal–organic frameworks with high porosity as materials for drug delivery. Adv Mater. 2011;23:5629–32. doi:10.1002/adma.201102538.
Goesten MG, Juan-Alcañiz J, Ramos-Fernandez EV, Gupta KBSS, Stavitski E, van Bekkum H, Gascon J, Kapteijn F. Sulfation of metal–organic frameworks: opportunities for acid catalysis and proton conductivity. J Catal. 2011;281:177–87. doi:10.1016/j.jcat.2011.04.015.
Serre C, Mellot-Draznieks C, Surblé S, Audebrand N, Filinchuk Y, Férey G. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science. 2007;315:1828–31. doi:10.1126/science.1137975.
Han J, Wang D, Du YH, Xi S, Chen Z, Yin S, Zhou T, Xu R. Polyoxometalate immobilized in MIL-101(Cr) as an efficient catalyst for water oxidation. Appl Catal A. 2016;521:83–9. doi:10.1016/j.apcata.2015.10.015.
Logvinenko VA, Dybtsev DN, Bolotov VA, Fedin VP. Thermal decomposition of inclusion compounds on the base of the metal–organic framework [Zn2(bdc)2(dabco)]. Part I. J Therm Anal Calorim. 2015;121:491–7.
Logvinenko VA, Aliev SB, Bolotov VA, Dybtsev DN, Fedin VP. Thermal (kinetic) stability of inclusion compounds on the basis of porous metal–organic frameworks. Dependence on the guest and framework properties. J Therm Anal Calorim. 2017;127:779–88. doi:10.1007/s10973-016-5398-6.
Logvinenko VA, Sapchenko SA, Fedin VP. Thermal decomposition of inclusion compounds on the base of the metal–organic framework [Zn4(dmf)(ur)2(ndc)4]. Part I. J Therm Anal Calorim. 2014;117:747–53.
Logvinenko VA, Sapchenko SA, Fedin VP. Thermal decomposition of inclusion compounds on the base of the metal–organic framework [Zn4(dmf)(ur)2(ndc)4]. Part II. J Therm Anal Calorim. 2016;123:697–702.
Zavakhina MS, Samsonenko DG, Virovets AV, Dybtsev DN, Fedin VP. Homochiral Cu(II) and Ni(II) malates with tunable structural features. J Solid State Chem. 2014;210:125–9.
Netzsch Thermokinetics. In: Netzsch Thermokinetics. http://www.netzsch-thermal-analysis.com/us/products-%20%20solutions/advanced-software/thermokinetics.html.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J Polym Sci. 1963;6:183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Ozawa T. Estimation of activation energy by isoconversion methods. Thermochim Acta. 1992;203:159–65.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand. 1966;70:478–523.
Opfermann J, Kaisersberger E. An advantageous variant of the Ozawa–Flynn–Wall analysis. Thermochim Acta. 1992;203:167–75.
Opfermann JR, Kaisersberger E, Flammersheim HJ. Model-free analysis of thermo-analytical data—advantages and limitations. Thermochim Acta. 2002;391:119–27.
Vyazovkin S. Model-free kinetics: staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Simon P. Single-step kinetics approximation employing nonarrhenius temperature functions. J Therm Anal Calorim. 2005;79:703–8.
Simon P. The single-step approximation: attributes, strong and weak sides. J Therm Anal Calorim. 2007;88:709–15.
Borchard HJ, Daniels F. The application of differential thermal analysis to the study of reaction kinetics. J Am Chem Soc. 1957;79:41–6.
Vyazovkin S, Burnham AK, Criado JM, Luis A, Perez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Chrissafis K, Di Lorenzo M-R, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol J-J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Berlin: Springer; 2015.
Simon P, Thomas P, Dubaj T, Cibulkova Z, Peller A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115:853–9.
Simon P, Dubaj T, Cibulkova Z. Equivalence of the Arrhenius and non-Arrhenian temperature functions in the temperature range of measurement. J Therm Anal Calorim. 2015;120:231–8.
Sestak J. Is the original Kissinger equation obsolete today: not obsolete the entire non-isothermal kinetics? J Therm Anal Calorim. 2014;117:3–7.
Galwey AK. What theoretical and/or chemical significance is to be attached to the magnitude of an activation energy determined for a solid-state decomposition by thermal analysis? J Therm Anal Calorim. 2006;86:267–86.
Roura P, Farjas J. Analytical solution for the Kissinger equation. J Mater Res. 2009;24:3095–8.
Holba P, Šesták J. Imperfections of Kissinger evaluation method and crystallization kinetics. Glass Phys Chem (translation from Russian journal). 2014;40:486–91.
Dubaj T, Cibulková Z, Šimon P. An incremental isoconversional method for kinetic analysis based on the orthogonal distance regression. J Comput Chem. 2015;36:392–8.
Logvinenko V. Stability and reactivity of coordination and inclusion compounds in the reversible processes of thermal dissociation. Thermochim Acta. 1999;340–341:293–9.
Logvinenko V, Drebushchak V, Pinakov D, Chekhova G. Thermodynamic and kinetic stability of inclusion compounds under heating. J Therm Anal. 2007;90:23–30.
Lomovsky O, Bychkov A, Lomovsky I, Logvinenko V, Burdukov A. Mechanochemical production of lignin-containing powder fuels from biotechnical industry waste: a review. Therm Sci. 2015;19:219–29.
Vasilyeva I, Logvinenko V. Contribution of chemical methods to the study of nanostructure of ultrafine and amorphous materials. Solid State Phenom. 2017;257:237–40.
Logvinenko VA, Makotchenko VG, Fedorov VE. Reactivity in combustion process for expanded graphites: influence of dimensional effect. Nanosyst Phys Chem Math (NANO. F & H & M). 2016;7:234–43.
Makotchenko VG, Pinakov DV, Logvinenko VA. The influence of dimensional effects on the composition and properties of polydicarbonfluoride. Chem Asian J. 2015;10:1761–7.
Chekhova GN, Pinakov DV, Shubin YV, Logvinenko VA. Structural rearrangements of the inclusion compound of fluorinated graphite with acetonitrile during isothermal deintercalation. J Therm Anal Calorim. 2017;128:349–55. doi:10.1007/s10973-016-5846-3.
Bushuev MB, Pishchur DP, Logvinenko VA, Gatilov YV, Korolkov IV, Shundrina IK, Nikolaenkova EB, Krivopalov VP. Mononuclear iron(II) complex: cooperativity, kinetics and activation energy of the solvent-dependent spin transition. Dalton Trans. 2016;45:107–20.
Dybtsev DN, Chun H, Kim K. Rigid and flexible: a highly porous metal–organic framework with unusual guest-dependent dynamic behavior. Angew Chem Int Ed. 2004;43:5033–6.
Hauptmann S, Graefe J, Remane H. Organische Chemie, 1 Auflage 1976, Deutscher Verlag für Grundstoffindustrie (3 Auflage 1991, Wiley-VCH, ISBN 3-527-30925-X).
Logvinenko VA. Stability of supramolecular compounds under heating. Thermodynamic and kinetic aspects. J Therm Anal Calorim. 2010;101:577–83.
Poling BE, Prausnitz JM, O’Connell JP. The properties of gases and liquids. New York: McGraw-Hill; 2001.
Mehio N, Dai S, Jiang D. Quantum mechanical basis for kinetic diameters of small gaseous molecules. J Phys Chem A. 2014;118:1150–4.
Acknowledgements
This work was partially supported by the Russian Foundation for Basic Research (Grant 14–03–00291).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Logvinenko, V., Zavakhina, M., Bolotov, V. et al. Some basic correlations in the thermal (kinetic) stability of inclusion compounds on the basis of microporous metal–organic frameworks. J Therm Anal Calorim 130, 335–342 (2017). https://doi.org/10.1007/s10973-017-6317-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6317-1