Abstract
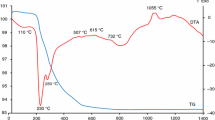
Novel environment-friendly yellow mixed oxide inorganic pigment from Bi2O3–ZnO–CeO2 system with the composition 23 mol% Bi2O3, 15 mol% ZnO and 62 mol% CeO2 was successfully synthesized by a conventional solid-state reaction method. Comprehensive analyses were carried out to characterize the develop pigment powder including simultaneous TG–DTA thermal analysis, colour properties and particle size distribution. The results demonstrated that the optimum calcination for pigment synthesis was located at a range 800–950 °C. The colour of the studied mixed oxide pigment is connected with the calcination condition. The substitution of Zn2+ changes the colour from orange to yellow. The colour of the obtained samples was dependent on the calcination condition and the particle size distribution. The most saturated yellow hue was obtained at the calcination temperature of 950 °C for 2 h in a furnace of pure air and after its application into organic binder in mass tone. The value C of this sample was approx. 65. The mixed oxide pigments were also evaluated from the standpoint of their particle size distribution. Bi2Ce2O7 is considered to be a non-toxic compound, and the other component (Zn2+ ions) is also the safe element. Therefore, the present mixed oxide could be an attractive candidate as a novel environment-friendly inorganic yellow pigment.



Similar content being viewed by others
References
Ozel E, Turan S. Production and characterisation of iron-chromium pigments and their interactions with transparent glazes. J Eur Ceram Soc. 2003;23:2097–104.
CPMA. Classification and chemical descriptions of the complex inorganic color pigments. 4th ed. Alexandria: Color Pigments Manufacturers Association, Inc; 2010.
Hunger K. Toxicology and toxicological testing of colorants. Rev Prog Color Relat Top. 2005;35(1):76–89.
Bae B, Tamura S, Imanaka N. Novel environmentally friendly inorganic yellow pigments based on gehlenite-type structure. Ceram Int. 2016;42:15104–6.
Sherman LM. Color formulator´selection guide. Plast Technol. 1996;42(5):48.
Steele JA, Lewis RA. In situ micro-Raman studies of laser-induced bismuth oxidation reveals metastability of β-Bi2O3 microislands. Opt Mater Express. 2014;4(10):2133–42.
Leontie L, Caraman M, Alexe M, Harnagea C. Structural and optical characteristics of bismuth oxide thin films. Surf Sci. 2002;507–510:480–5.
Tran TB, Navrotsky A. Energetics of disorder and ordered rare earth oxide-stabilized bismuth oxide ionic conductors. Phys Chem Chem Phys. 2014;16(5):2331–7.
Drache M, Roussel P, Wignacourt JP. Structures and oxide mobility in Bi–Ln–O materials: heritage of Bi2O3. Chem Rev. 2007;107(1):80–96.
Mehring M. From molecules to bismuth oxide-based materials: potential homo- and heterometallic precursors and model compounds. Coord Chem Rev. 2007;251(7–8):974–1006.
Atou T, Fagir H, Kikuchi M, Chiba H, Syono Y. A new high-pressure phase of bismuth oxide. Mater Res Bull. 1998;33(2):289–92.
Narang SN, Patel ND, Kartha VB. Infrared and Raman spectral studies and normal modes of α-Bi2O3. J Mol Struct. 1994;327(2–3):221–35.
Serena S, De La Rubia MA, Caballero AC, Caballero YA. Thermodynamic study of the rich-Bi2O3 region of the Bi2O3–ZnO system. Bol Soc Esp Cerám Vidr. 2006;45(3):150–3.
Kašpar J, Fornasiero P, Graziani M. Use of CeO2-based oxides in the three-way catalysis. Catal Today. 1999;50:285–98.
Trovarelli A. Catalytic properties of ceria and CeO2-containing materials. Catal Rev Sci Eng. 1996;38:439–520.
Masui T, Minami K, Koyabu K, Imanaka N. Synthesis and characterization of new promoters based on CeO2–ZrO2–Bi2O3 for automotive exhaust catalysts. Catal Today. 2006;117:187–92.
Zheng X, Zhang X, Fang Z, Wang X, Wang S, Wu S. Characterization and catalysis studies of CuO/CeO2 model catalysts. Catal Commun. 2006;7:701–4.
Brisse F, Knop O. Pyrochlores. II. An investigation of La2Ce2O7 by neutron diffraction. Can J Chem. 1976;45(6):609–14.
Völz HG. Industrial color testing: fundamentals and techniques. 2nd ed. Weinheim: Wiley; 2002.
Těšitelová K, Šulcová P. Synthesis and study of Bi2Ce2O7 as inorganic pigment. J Therm Anal Calorim. 2016;125(3):1047–52.
Šulcová P, Trojan M. Thermal synthesis and properties of the (Bi2O3)1−x(Ho2O3)x pigments. J Therm Anal Calorim. 2006;83:557–9.
Šulcová P, Večeřa J, Strnadlová L. Study of doped CeO2 prepared by different synthesis. J Therm Anal Calorim. 2012;108:519–23.
Acknowledgements
The work was supported by Grant Project of the Czech Science Foundation No. 16-06697S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Těšitelová, K., Šulcová, P. Synthesis and study of mixed oxide inorganic pigment from Bi2O3–ZnO–CeO2 system. J Therm Anal Calorim 130, 57–62 (2017). https://doi.org/10.1007/s10973-017-6316-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6316-2