Abstract
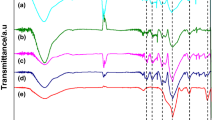
Two different methods were used for preparation of novolac phenolic resin nanohybrids containing carbon nanotube and silica/siloxane moieties. Oxidized carbon nanotube was modified with furfuryl alcohol and 3-(trimethoxysilyl)propyl methacrylate (MPS) to obtain CNTFASi. Carbon nanotube xerogel (CNTFAX) was then obtained by incorporation of CNTFASi into silica/siloxane network by using tetraethyl orthosilicate (TEOS). The first nanohybrid was prepared by addition of CNTFAX into the novolac phenolic resin matrix. Incorporation of (3-glycidyloxypropyl) trimethoxysilane-modified novolac phenolic resin, CNTFASi, and TEOS into silica/siloxane network results in the second nanohybrid. These two types of nanohybrids were compared from the viewpoint of thermal properties. Successful modification of carbon nanotube and novolac phenolic resin was proved by Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, and thermogravimetric analysis results. Molar ratios of furfuryl alcohol in CNTFA and MPS in CNTFASi are 597 and 108 μmol g−1, respectively. Formation of silica/siloxane xerogel network in CNTFAX was evaluated by X-ray diffraction, scanning electron microscopy, and transmission electron microscopy. The results showed that nanotubes with smooth surface are incorporated into the spongy silica/siloxane matrix. Char residue of CR is decreased from 58.2 to 50.6% by the addition of only 4 mass% of CNTFAX. Finally, char residue of 71.1% for the nanohybrid of modified novolac resin with 4 mass% of CNTFASi shows 12.8% increase in char residue with respect to the neat cured resin.








Similar content being viewed by others
References
Rapacz-Kmita A, Gajek M, Dudek M, Stodolak-Zych E, Szaraniec B, Lach R. Thermal, structural and mechanical analysis of polymer/clay nanocomposites with controlled degradation. J Therm Anal Calorim. 2016. doi:10.1007/s10973-016-5771-5.
Patsidis AC, Kalaitzidou K, Psarras GC. Graphite nanoplatelets/polymer nanocomposites: thermomechanical, dielectric, and functional behavior. J Therm Anal Calorim. 2014;116:41–9.
Liu Y, Jing X. Pyrolysis and structure of hyperbranched polyborate modified phenolic resins. Carbon. 2007;45:1965–71.
Xu P, Jing X. Pyrolysis of hyperbranched polyborate modified phenolic resin. Polym Eng Sci. 2010;50:1382–8.
Chiang CL, Ma CCM, Wu DL, Kuan HC. Preparation, characterization, and properties of novolac-type phenolic/SiO2 hybrid organic–inorganic nanocomposite materials by sol–gel method. J Polym Sci Part A Polym Chem. 2003;41:905–13.
Lin JM, Ma CC, Wang FY, Wu HD, Kuang SC. Thermal, mechanical, and morphological properties of phenolic resin/silica hybrid creamers. J Polym Sci Part B Polym Phys. 2000;38:1699–706.
Roghani-Mamaqani H, Haddadi-Asl V, Mortezaei M, Khezri K. Furfuryl alcohol functionalized graphene nanosheets for synthesis of high carbon yield novolak composites. J Appl Polym Sci. 2014;131:40273.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M. Functionalization of carbon nanotubes by furfuryl alcohol moieties for preparation of novolac phenolic resin composites with high carbon yield values. Colloid Polym Sci. 2015;293:3623–31.
Sobani M, Haddadi-Asl V, Salami-Kalajahi M, Roghani-Mamaqani H, Mirshafiei-Langari SA, Khezri K. “Grafting through” approach for synthesis of polystyrene/silica aerogel nanocomposites by in situ reversible addition-fragmentation chain transfer polymerization. J Sol Gel Sci Technol. 2013;66:337–44.
Mirshafiei-Langari SA, Haddadi-Asl V, Roghani-Mamaqani H, Sobani M, Khezri K. Synthesis of hybrid free and nanoporous silica aerogel-anchored polystyrene chains via in situ atom transfer radical polymerization. Polym Compos. 2013;34:1648–54.
Sobani M, Haddadi-Asl V, Mirshafiei-Langari SA, Salami-Kalajahi M, Roghani-Mamaqani H, Khezri K. A kinetics study on the in situ reversible addition-fragmentation chain transfer and free radical polymerization of styrene in presence of silica aerogel nanoporous particles. Des Monomers Polym. 2014;17:245–54.
Mirshafiei-Langari SA, Haddadi-Asl V, Roghani-Mamaqani H, Sobani M, Khezri K. In situ atom transfer radical polymerization of styrene in the presence of nanoporous silica aerogel: kinetic study and investigation of thermal properties. J Polym Res. 2013;20:163.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M. Organic-inorganic nanohybrids of novolac phenolic resin and carbon nanotube: high carbon yields by using carbon nanotube aerogel and resin incorporation into aerogel network. Microporous Mesoporous Mater. 2016;224:58–67.
Noparvar-Qarebagh A, Roghani-Mamaqani H, Salami-Kalajahi M. Novolac phenolic resin and graphene aerogel organic-inorganic nanohybrids: high carbon yields by resin modification and its incorporation into aerogel network. Polym Degrad Stab. 2016;124:1–14.
Roghani-Mamaqani H, Haddadi-Asl V, Ghaderi-Ghahfarrokhi M, Sobhkhiz Z. Reverse atom transfer radical polymerization of methyl methacrylate in the presence of Azo-functionalized carbon nanotubes: a grafting from approach. Colloid Polym Sci. 2014;292:2971–81.
Roghani-Mamaqani H, Haddadi-Asl V, Sobhkhiz Z, Ghaderi-Ghahfarrokhi M. Grafting poly (methyl methacrylate) from azo-functionalized graphene nanolayers via reverse atom transfer radical polymerization. Colloid Polym Sci. 2015;293:735–50.
Roghani-Mamaqani H, Haddadi-Asl V. In-plane functionalizing graphene nanolayers with polystyrene by atom transfer radical polymerization: grafting from hydroxyl groups. Polym Compos. 2014;35:386–95.
Roghani-Mamaqani H, Haddadi-Asl V, Khezri K, Zeinali E, Salami-Kalajahi M. In situ atom transfer radical polymerization of styrene to in-plane functionalize graphene nanolayers: grafting through hydroxyl groups. J Polym Res. 2014;21:333.
Roghani-Mamaqani H, Haddadi-Asl V, Khezri K, Salami-Kalajahi M. Edge-functionalized graphene nanoplatelets with polystyrene by atom transfer radical polymerization: grafting through carboxyl groups. Polym Int. 2014;63:1912–23.
Roghani-Mamaqani H, Haddadi-Asl V, Khezri K, Salami-Kalajahi M, Najafi M. Kinetic study of styrene atom transfer radical polymerization from hydroxyl groups of graphene nanoplatelets: heterogeneities in chains and graft densities. Polym Eng Sci. 2015;55:1720–32.
Roghani-Mamaqani H, Khezri K. A grafting from approach to graft polystyrene chains to the surface of graphene nanolayers by RAFT polymerization: various graft densities from hydroxyl groups. Appl Surf Sci. 2016;360:373–82.
Jafarzadeh S, Haddadi-Asl V, Roghani-Mamaqani H. Nanofibers of poly (hydroxyethyl methacrylate)-grafted halloysite nanotubes and polycaprolactone by combination of RAFT polymerization and electrospinning. J Polym Res. 2015;22:123.
Yuan FY, Zhang HB, Li X, Ma HL, Li XZ, Yu ZZ. In situ chemical reduction and functionalization of graphene oxide for electrically conductive phenol formaldehyde composites. Carbon. 2014;68:653–61.
Yuen SM, Ma CCM, Chiang CL, Teng CC, Yu YH. Poly(vinyltriethoxysilane) modified MWCNT/polyimide nanocomposites: preparation, morphological, mechanical, and electrical properties. J Polym Sci Part A Polym Chem. 2008;46:803–16.
Yang H, Shan C, Li F, Han D, Zhang Q, Niu L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem Commun. 2009;26:3880–2.
Li W, Tang XZ, Zhang HB, Jiang ZG, Yu ZZ, Du XS, Mai YW. Simultaneous surface functionalization and reduction of graphene oxide with octadecylamine for electrically conductive polystyrene composites. Carbon. 2011;49:4724–30.
Roghani-Mamaqani H, Haddadi-Asl V, Khezri K, Salami-Kalajahi M, Najafi M, Sobani M, Mirshafiei-Langari SA. Confinement effect of graphene nanoplatelets on atom transfer radical polymerization of styrene: grafting through hydroxyl groups. Iran Polym J. 2015;24:51–62.
Cochet M, Maser WK, Benito AM, Callejas MA, Martinez MT, Benoit JM, Schreiber J, Chauvet O. Synthesis of a new polyaniline/nanotube composite: in situ polymerisation and charge transfer through site-selective interaction. Chem Commun. 2001;16:1450–1.
Yao X, Wu H, Wang J, Qu S, Chen G. Carbon Nanotube/poly(methyl methacrylate) (CNT/PMMA) composite electrode fabricated by in situ polymerization for microchip capillary electrophoresis. Chem Eur J. 2007;13:846–53.
Lei Z, Yan Y, Feng J, Wu J, Huang G, Li X, Xing W, Zhao L. Enhanced power factor within graphene hybridized carbon aerogels. RSC Adv. 2015;5:25650–6.
Qian Y, Wei P, Jiang P, Liu J. Preparation of halogen-free flame retardant hybrid paraffin composites as thermal energy storage materials by in situ sol–gel process. Sol Energy Mater Sol Cells. 2012;107:13–9.
Wang Z, Wei P, Qian Y, Liu J. The synthesis of a novel graphene-based inorganic–organic hybrid flame retardant and its application in epoxy resin. Compos B. 2014;60:341–9.
Cheng WY, Wang CC, Lu SY. Graphene aerogels as a highly efficient counter electrode material for dye-sensitized solar cells. Carbon. 2013;54:291–9.
Wu C, Huang X, Wang G, Wu X, Yang K, Li S, Jiang P. Hyperbranched-polymer functionalization of graphene sheets for enhanced mechanical and dielectric properties of polyurethane composites. J Mater Chem. 2012;22:7010–9.
Ngo VG, Bressy C, Leroux C, Margaillan A. Synthesis of hybrid TiO2 nanoparticles with well-defined poly(methyl methacrylate) and poly(tert-butyldimethylsilyl methacrylate) via the RAFT process. Polymer. 2009;50:3095–102.
Xu P, Jing X. High carbon yield thermoset resin based on phenolic resin, hyperbranched polyborate, and paraformaldehyde. Polym Adv Technol. 2011;22:2592–5.
Wang S, Jing X, Wang Y, Si J. High char yield of aryl boron-containing phenolic resins: the effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polym Degrad Stab. 2014;99:1–11.
Hsiue GH, Shiao SJ, Wei HF, Kuo WJ, Sha YA. Novel phosphorus-containing dicyclopentadiene-modified phenolic resins for flame-retardancy applications. J Appl Polym Sci. 2001;79:342–9.
Cui J, Yan Y, Liu J, Wu Q. Phenolic resin-MWNT nanocomposites prepared through an in situ polymerization method. Polym J. 2008;40:1067–73.
Liu L, Ye Z. Effects of modified multi-walled carbon nanotubes on the curing behavior and thermal stability of boron phenolic resin. Polym Degrad Stab. 2009;94:1972–8.
Acknowledgements
Iran’s National Elites Foundation is greatly appreciated for its financial support (Grant Number: 15/76508).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Noparvar-Qarebagh, A., Roghani-Mamaqani, H., Salami-Kalajahi, M. et al. Nanohybrids of novolac phenolic resin and carbon nanotube-containing silica network. J Therm Anal Calorim 128, 1027–1037 (2017). https://doi.org/10.1007/s10973-016-5970-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5970-0