Abstract
New segmented polyurethanes (SPURs) were synthesized by one-step melt polyaddition from a poly(oxytetramethylene)diol of \(\overline{M}_{\text n}\) = 1000 g mol−1 (PTMO) or a poly(hexamethylene carbonate)diol of \(\overline{M}_{\text n}\) = 860 g mol−1 (PHCD) as soft segments, 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethylcyclohexane (IPDI), and 2,2′-methylenebis[(4,1-phenylene)methylenesulfanediyl]diethanol (diol E) as an unconventional chain extender. Furthermore, some of SPURs were modified by the addition of a carboxylic group by means of 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoic acid. The effects of the kind and amount of the polymer diol and chain extender used on the structure and properties of the polymers were studied. The polymers were examined by attenuated total reflection Fourier transform infrared spectroscopy, gel permeation chromatography, thermogravimetric analysis (TG), TG–FTIR, differential scanning calorimetry (DSC), Shore A/D hardness and tensile testing. The obtained SPURs were amorphous, colourless, high molar mass materials which showed elastomeric or plastic properties. Their T g s were in the range of −51 to 29 °C. It was observed that the polymers with a PHCD demonstrated a better segmental miscibility (higher T g s), as well as greater hardness and tensile strengths, but smaller elongations at break than PTMO-based ones. All of the polymers exhibited a relatively good thermal stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyurethane elastomers are a very interesting type of polyurethane materials. They are now widely used due to their unique properties, such as an outstanding mechanical strength, good chemical resistance and excellent elasticity. In recent years, the importance of thermoplastic polyurethane elastomers (TPUs) has grown as a result of a less complicated and less expensive production process compared to vulcanized elastomers as well as the possibility of their recycling. TPUs are polymers that show properties characteristic of elastomers in the normal conditions of use getting plasticized when heated. This means that they may be processed using methods typical of thermoplastics, i.e. extrusion, calendaring or injection [1–3].
Typical TPUs are multiblock copolymers consisting of alternating flexible “soft” segments derived from aliphatic linear polymer diols and “hard” segments formed from diisocyanates and short-chain diols.
TPUs are polyaddition reaction products involving aromatic (mainly 1,1′-methylenebis (4-isocyanatobenzene) (MDI)) or aliphatic diisocyanates (predominantly 1,1′-methylenebis (4-isocyanatocyclohexane) (HMDI) and 1,6-diisocyanatohexane (HDI)), aliphatic linear polymer diols (polyetherdiols, polyesterdiols or polycarbonate diols) as well as the chain extenders (usually butane-1,4-diol), which in the case of less reactive substrates require the presence of a catalyst [1–3].
Aromatic diisocyanate-based TPUs generally bear superior mechanical properties due to a strong cohesion force between the hard segment chains. However, the aromatic diisocyanate-based TPUs possess serious defects, such as change of colour or decrease in thermal and mechanical properties against ultraviolet and visible light and heat [1, 3, 4]. In contrast, the aliphatic diisocyanate-based TPUs show no change of colour in the same condition [3].
This paper is a continuation of research on the new TPUs derived from aliphatic–aromatic sulphur-containing chain extenders as well as derivatives of diphenylmethane [5–13], diphenylethane [14–16], benzophenone [17–19], diphenyl ether [20] and diphenyl sulphide [21, 22]. Based on the test results, it can be concluded that the introduction of sulphur atoms into the structure of a polymer has increased their adhesive strength [21] and refractive index [11, 12]. In addition, antibacterial properties against gram-positive bacteria were indicated [12].
In this work, we present both a synthesis and characterization of 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethylcyclohexane (IPDI)-based segmented polyurethanes (SPURs) with polyether and polycarbonate soft segments, i.e. poly(oxytetramethylene)diol of \(\overline{M}_{\text n}\) = 1000 g mol−1 (PTMO) or poly(hexamethylene carbonate)diol of \(\overline{M}_{\text n}\) = 860 g mol−1 (PHCD) and an unconventional chain extender, i.e. 2,2′-methylenebis[(4,1-phenylene)methylenesulfanediyl]diethanol (diol E). The content of hard segments was contained within 30–60 mass%. Additionally, SPURs with the hard content of 50 mass% were modified by the addition of a carboxylic group by means of 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoic acid (DMPA). Because the newly obtained SPURs incorporate sulphur atoms in their structure, they can exhibit improved antimicrobial activity as well as both optical and adhesive properties [23, 24]. On the other hand, the introduction of functional groups into the polymer structure, such as sulphonic and carboxylic ones, makes it possible to improve the biocompatibility of these materials and therefore apply them in the production of various medical appliances [25, 26]. Additionally, their presence makes it possible to obtain ionomers that can be applied in the production of, inter alia, coating materials [25–31].
This work also gives a characterization of the newly obtained regular polyurethane (R-PUR) based on IPDI and diol E, building the hard segment in TPUs.
Experimental
Materials
The diol E (m.p. = 77–78 °C) was obtained by the condensation reaction of [methylenedi(4,1-phenylene)]dimethanethiol with 2-chloroethanol in 10 % aqueous solution of sodium hydroxide [10]. PTMO and PHCD were purchased from Sigma-Aldrich (St. Louis, USA). Before being used, the PTMO and PHCD were heated at 90 °C in vacuo for 10 h. IPDI and dibutyltin dilaurate (DBTDL) from Merck Schuchardt (Hohenbrunn, Germany) and DMPA from Sigma-Aldrich (Steinheim, Germany) were used as received. The polymerization solvent, N,N-dimethylformamide (DMF), with a water content of less than 0.01 %, was purchased from Sigma-Aldrich (Steinheim, Germany) and was used as received.
Measurement methods
Attenuated total reflection–Fourier transform infrared (ATR–FTIR) spectra were obtained with a FTIR TENSOR 27 (Bruker, Germany) spectrophotometer using thin films or powder (for R-PUR). Spectra were recorded from 4000 to 600 cm−1 averaging 32 scans with a resolution of 4 cm−1.
Elemental analysis was performed with a PerkinElmer CHN 2400 analyser (Norwalk, USA).
Reduced viscosities (η red, dL g−1) of 0.5 % polymer solution in 1,1,2,2-tetrachloroethane (TChE) were measured in an Ubbelohde viscometer (Gliwice, Poland) at 25 °C.
The number (\(\overline{M}_{\text n}\)) and mass (\(\overline{M}_{\text w}\)) average molar mass (g mol−1), and the molar mass dispersity (Ð M, Ð M = \(\overline{M}_{\text w}\) / \(\overline{M}_{\text n}\)) of the segmented polyurethanes were determined by gel permeation chromatography (GPC) performed on a Viscotek GPCMax (USA) equipped with triple detector array TDA305. The eluent was tetrahydrofuran (THF), the flow rate was 1 mL min−1, the operation temperature was set to be 35 °C, and the molar mass was calibrated with polystyrene standards.
The content of carboxylic group of the carboxylate SPURs was determined by acid–base titration as follows. A given mass (about 3 g) of the sample was dissolved in 200 cm3 of hot DMF, and two drops of phenolphthalein solution as indicator were added with agitation. The resulting solution was titrated with 0.1 M KOH in isopropanol solution.
Thermogravimetric analysis (TG) was performed on a MOM 3 427 derivatograph (Paulik, Paulik and Erdey, Budapest, Hungary) in the range of 20–1000 °C in air atmosphere, at the heating rate of 10 °C min−1. All TG measurements were taken in Al2O3. As a reference, empty Al2O3 crucible was applied. Sample masses about 100 mg were used.
TG–FTIR was carried out with a Netzsch STA 449 F1 Jupiter thermal analyser (Germany). In a typical procedure, ca. 10 mg of the sample was heated from 40 up to 700 °C with a heating rate of 10 °C min−1 in open Al2O3 crucible (mass of 160 ± 1 mg) under inert conditions (helium, flow rate 20 mL min−1). As a reference, empty Al2O3 crucible was applied. The composition of the gas evolved during the decomposition process was analysed by a Bruker Tensor 27 FTIR spectrometer (Germany) coupled online to a Netzsch STA instrument by Teflon transfer line with 2 mm diameter heated to 200 °C. The FTIR spectra were recorded in the spectral range of 600–4000 cm−1 with 16 scans per spectrum at 4 cm−1 resolution.
Differential scanning calorimetry (DSC) experiments were performed with a Netzsch 204 calorimeter (Germany). All DSC measurements were taken in aluminium pans with pierced lid (mass of 40 ± 1 mg). As a reference, empty aluminium crucible was applied. Sample masses of about 10 mg were used. The sample pan was placed in the calorimeter at ~25 °C and then subjected to the following time–temperature program: (1) cooling and isotherm for 3 min at −100 °C; (2) heating to 200 °C; (3) cooling to −100 °C; and (4) heating to 200 °C. The reported transitions were taken from first and second heating scans. The scans were performed at the heating/cooling rate of 10 °C min−1 under nitrogen atmosphere (flow = 30 cm3 min−1). Glass transition temperatures (T g s) for the polymer samples were taken as the inflection point on the curves of the heat capacity changes.
The hardness of the SPURs was measured by the Shore A/D method on a Zwick 7206/H04 hardness tester (Germany) at 23 °C. The values were taken after 15 s.
Tensile testing was performed on a Zwick/Roell Z010 tensile testing machine (Germany) according to Polish Standard PN-81/C-89034 at the speed of 100 mm min−1 at 23 °C; the tensile test pieces 1 mm thick and 6 mm wide (for the section measured) were cut from the pressed sheet. Press moulding was done with a Carver hydraulic press (USA) at 120–160 °C under 10–30 MPa pressure.
Polymer synthesis
R-PUR
R-PUR was prepared by the solution polymerization of an equimolar amount (0.01 mol) of diol E and IPDI (DMF, concentration ~20 mass%); this was carried out under dry nitrogen for 4 h at 85 °C in the presence of a catalytic amount of DBTDL (about 0.03 g). The polymer precipitated and was then washed with distilled water. The obtained material was dried at 100 °C in vacuum.
An FTIR scan of the synthesized material showed the following absorption peaks (cm−1): 1700 (H-bonded C=O stretching), 1509 (N–H bending) and 3327 (N–H stretching) of the urethane group; 3020 (C–H stretching of benzene ring); 816 (C–H bending of 1,4-substituted benzene ring); 1461 (C–H bending), 772 (C–C bending) of cyclohexane ring; 2949 and 2914 (asymmetric and symmetric C–H stretching, respectively) of CH2.
Elemental analysis
Calcd for C31H42N2O4S2: C 64.94 %, H 7.29 %, N 5.31 %; found: C 65.23 %, H 7.41 %, N 4.91 %.
SPURs
SPURs with the hard segment contents of ~30, 40, 50 and 60 mass% were prepared, according to Scheme 1, by the one-step melt polyaddition process from IPDI, diol E, PTMO or PHCD at the NCO/OH molar ratio of 1.05.
The general procedure for the synthesis of SPURs by this method was as follows. PTMO or PHCD and diol E or diol E and DMPA (0.01 mol together) and IPDI (0.0105 mol) were heated with stirring under dry nitrogen to 95 °C in an oil bath. A catalytic amount of DBTDL (about 0.03 g) was added to the clear melt formed, and polymerization rapidly began at vigorous stirring. The reaction temperature was gradually raised to 130 °C, and the colourless rubber-like product formed was additionally heated at this temperature for 2 h.
SPURs with the hard content of 50 mass% were modified by the addition of a carboxylic group by means of DMPA. Carboxylated SPURs were prepared in a similar way to non-carboxylated SPURs, except that 20 and 40 % of diol E was replaced with an ionic chain extender DMPA. These polymers were designated as D20 and D40, respectively.
An FTIR scan of the PTMO-based SPURs showed the following absorption peaks (cm−1): 1701–1653 (C=O stretching); 3368–3329 (N–H stretching) and 1535–1531 (N–H bending) of the urethane group; 1112–1106 (C–O stretching of the ether group); 1467–1447 (C–H bending of the cyclohexane ring); 2943–2941 and 2859–2857 (asymmetric and symmetric C–H stretching of CH2, respectively); 1368–1366 symmetric C–H bending of the CH3 group.
An FTIR scan of the PHCD-based SPURs showed the following absorption peaks (cm−1): 1745–1741 (non-bonded C=O stretching of the carbonate group); 1721–1717 (non-bonded C=O stretching of the urethane group and H-bonded C=O stretching of the carbonate group); 1535–1528 (N–H bending) and 3368–3329 (N–H stretching) of the urethane group; 1263–1239 and 960–959 (asymmetric and symmetric C–O stretching of the carbonate group, respectively); 793–790 (C–O bending of the carbonate group); 1464–1447 (C–H bending of the cyclohexane ring); 2944–2941 and 2864–2857 (asymmetric and symmetric C–H stretching of CH2, respectively); 1369–1365 symmetric C–H bending of the CH3 group.
Results and discussion
Polymer characterization
The new SPURs were colourless, high transparent solids. All synthesized polymers were insoluble in DMSO, but easily dissolved in THF and TChE in room temperature, N-methyl-2-pyrrolidone (NMP) and N,N-dimethylacetamide (DMAc) at room or elevated temperature, and some of the polymers were partially soluble in DMF. Generally, the solubility of carboxylated SPURs decreased with increase in carboxyl groups in polymer (Table 1, Fig. 1).
In the case of other solvents, polymers dissolved incompletely or only after being heated. SPURs with the content of 20 mol% DMPA dissolved very well in DMF, NMP and DMAc, while those with the DMPA content of 40 mol% swelled in these solvents. Therefore, it can be concluded that with the increase in DMPA, the solubility of SPURs in the said solvents decreased.
The η zred values for SPURs (contained in Table 2) ranged from 0.70 to 6.74 dL g−1. This indicates their high molar masses (as verified using the GPS method). Greater η zred values were shown by polymers with a polyether soft segment (except for 60P) than polymers derived from PHCD. Based on the values presented in Table 2, it can be stated that the viscosity of carboxylated SPURs increases with the increase in the content of DMPA, whereas the more pronounced increase was observed in the case of SPURs derived from PHCD. The viscosity of carboxylated SPURs synthesized with the use of PTMO is greater than the property showed by those with PHCD. When analysing the impact of the polymer DMPA additive, it can be stated that in most cases a 20 mol% DMPA additive resulted in a decrease in viscosity, while a 40 mol% DMPA additive resulted in its significant increase.
When comparing the \(\overline{{M_{\text n} }}\) and \(\overline{{M_{\text w} }}\) values for both soft segments, it can be stated that, in general, greater values were shown by the polymers derived from PTMO. A \(\overline{{M_{\text w} }} /\overline{{M_{\text n} }}\) relationship indicative of molar mass dispersity for the polymers obtained ranged from 1.12 to 2.28, and it was at a relatively low level for polymers obtained in an alloy [1].
The results obtained in the course of acid-based titration revealed a lower content of the COOH groups in all carboxylated SPURs in relation to theoretical values. This may be due to the fact that some carboxyl groups contained in DMPA reacted with diisocyanate –NCO groups [26, 27].
Thermal properties
The thermal stability of SPURs was obtained using a TG analysis conducted in air atmosphere. The temperatures of 5 % (T5), 10 % (T10) and 50 % (T50) mass loss were designated from TG curves, while temperatures of a maximum rates of mass loss (Tmax) were determined from DTG curves. The values of the temperatures determined are presented in Table 3.
TG
Temperatures of a 5 % mass loss for non-carboxylated SPURs ranged from 305 to 325 °C and from 285 to 295 °C for carboxylated SPURs. On this basis, it can be concluded that DMPA addition resulted in the decrease in thermal stability of the polymers obtained. In general, the polymers derived from PTMO showed a higher T 5 than the corresponding polymers based on PHCD. This results from a better stability of PTMO as a soft segment than that shown by PHCD.
When analysing the course of DTG curves, it can be stated that the decomposition of the polymers is a two-step process. In the case of polymers derived from PTMO, the first step (a sharp peak with a maximum in the range of 392–405 °C) may be due to the decomposition of urethane, sulphide and ether linkages, while the second step (temperatures in the range of 540–586 °C) may be connected with the oxidizing processes of the solid products from the first step. In the case of polymers based on PHCD, the first step (a wide peak in the range of 360–376 °C) was due to the decomposition of urethane, sulphide and carbonate linkages, while the second step (temperatures in the range of 535–590 °C), as for PTMO derivatives, may be associated with the oxidizing processes of the solid products from the first step [6, 7].
TG–FTIR
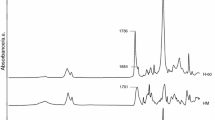
In order to better describe the process of decomposition of the polymers obtained and to determine volatile products, TG–FTIR analysis was conducted for R-PUR and the following SPURs: 50P and 50C. The process was conducted in helium atmosphere. Figure 2 depicts the DTG and TG curves of the said polymers obtained in helium atmosphere, while Fig. 3 presents FTIR spectra of the products of decomposition of the polymers in their T max.
The FTIR spectra of gaseous products of decomposition obtained in the course of the first step of R-PUR decomposition (T max = 355 °C) showed very strong double absorption bands at 2072 and 2047 cm−1, typical of both asymmetric and symmetric stretching vibrations C=O and a small band at 868 cm−1 connected with stretching vibrations C=S in carbonyl sulphide. The presence of absorption bands at 2359 and 669 cm−1 indicated the formation of carbon dioxide. Moreover, the FTIR spectra showed the absorption bands typical for aromatic hydrocarbons at 3040 cm−1 (stretching vibrations C–H of a benzene ring) and at 1508 cm−1 (stretching vibrations C=C of a benzene ring). The presence of aliphatic compounds was confirmed by the absorption bands at 2929–2890 cm−1 connected with both asymmetric and symmetric stretching vibrations of methylene and methyl groups. In addition to the above-mentioned absorption bands, there were bands typical of alcohols on the FTIR spectra: at 3750–3550 cm−1 (stretching vibrations –OH); at a 1050 cm−1 (stretching C–OH), as well as bands typical of primary amines: at 3340 cm−1 (stretching vibrations N–H) and at 1666 cm−1 (bending N–H) and aldehydes: at 2820 and 2714 cm−1 (stretching vibrations C–H) and at 1730 cm−1 (stretching vibrations C=O). Moreover, the present absorption band at 1050 cm−1 suggests that the product of decomposition was also aliphatic ethers.
R-PUR decomposition took place based on the mechanisms 1 (formation of isocyanates and alcohols), 2 (formation of primary amines) and 4 generating COS [5].
There are three peaks visible on the DTG curve obtained for polymer 50P: 366 and 379 °C (corresponding to the decomposition of urethane and sulphide linkages) and 393 °C (corresponding to the decomposition of ether linkage), while there is one sharp peak visible on the DTG curve obtained for polymer 50C at 363 °C (corresponding to the decomposition of urethane, sulphide and carbonate linkages) [6, 7]. On both curves, there are no other high temperature peaks which would be visible in the case of an analysis conducted in air atmosphere.
On each FTIR spectrum of gaseous decomposition products, there were visible absorption bands at 2071 and 2049 cm−1 typical of both asymmetric and symmetric vibrations C=O in carbonyl sulphide, absorption bands at 2376–2349 cm3 and 669 cm−1 connected with C=O vibrations in carbon dioxide, absorption bands at 2935–2865 cm−1 typical of the vibrations of both methyl and methylene groups as well as a band at 2274 cm−1 typical of asymmetric vibrations of –NCO group. The presence of those bands on the spectrum results from the decomposition of hard segments.
In the case of polymer 50P during the first stage (366 °C) only the above-presented absorption bands were visible. This suggests that at this temperature a decay of the hard segment took place according to the mechanisms 1, 2 and 4. At higher temperature (393 °C), the intensity of a band, which is characteristic of COS, almost disappears, while the band’s intensity at 1111 cm−1 characteristic of stretching vibrations C–O ether group and bands at 2938–2862 cm−1 characteristic of vibrations of both methyl and methylene groups. There are, in turn, absorption bands at 2822 and 2710 cm−1 as well as at 1726 cm−1, which were connected with, respectively, stretching vibrations C–H and stretching vibrations C=O in aldehydes. There appeared also a small peak at approx. 2170 cm−1, connected with stretching vibrations C–O in carbon monoxide [5]. It indicated that at that temperature the decay of the polyether soft segment took place and its products were aldehydes and aliphatic ethers.
In the case of polymer 50C, in addition to the absorption bands occurring during the decay of hard segments (according to mechanism corresponding to 50P), absorption bands were visible at 1260 cm−1, characteristic of the stretching vibrations C–O of carbonate group and at 1745 cm−1, characteristic of vibrations C=O of carbonate group. The presence of absorption band at 1066 cm−1 (characteristic of the stretching vibrations C–OH) and at approx. 3734 cm−1 (characteristic of the stretching vibrations O–H) suggested that the product of decomposition of that polymer was also alcohols and ethers (absorption band at 1066 cm−1 characteristic of the stretching vibrations of the C–O ether group). A small absorption band at 916 cm−1 indicated that during the decay of a polymer an ethylene oxide was created.
DSC
DSC measurements were taken in the course of two heating cycles at the temperature ranging from −100 to 200 °C. Table 3 contains numeric data determined from DSC curves from the first and second heating cycles. Figure 4 shows the DSC curves of the selected polymers.
On the DSC curves of all polymers, only glass transitions were visible, there are no endothermic peaks. It indicates that the obtained polymers were amorphous ones. The T g values for non-carboxylated SPURs ranged from −51 to 29 °C and for carboxylated SPURs between −33 and 20 °C. The analysis of DSC data from Table 3 revealed that T g increased with the growth of the hard segment contents in SPURs. In the case of carboxylated SPURs, the increase in DMPA content in a polymer resulted in a decrease of T g. When the type of a soft segment is a criterion for comparison, it is evident that a lower T g as well as a lower difference between T g value for a pure soft segment (PTMO-1000: −77 °C, PHCD: −68 °C) [5] and an appropriate polymer, and at the same time better microphase separation, was revealed by polymers derived from PTMO.
The data contained in Table 3 confirm that some polymer PHCD derivatives revealed T g at approx. room temperature, which means that they were on the border of plastomers and elastomers. These polymers were 50C, 60C and PHCD-based carboxylated SPURs.
The analysis concerning the addition of DMPA to a polymer shows that in the case of a series of polymers with a polyether soft segment, the modified polymers had lower T g values. In a series of carboxylated SPURs with PHCD, the differences are not so visible.
Mechanical properties
The mechanical properties (such as module of elasticity, tensile strength, elongation at break) and hardness based on A and D scales of the Shore durometer for each polymer following their compression at a temperature in the range of 70–140 °C under a load of 10–30 MPa are listed in Table 4, while stress–strain curves for SPURs, PHCD derivatives, are presented in Fig. 5.
When analysing data contained in Table 3, it can be concluded that with an increasing hard segment in non-carboxylated SPURs their hardness increased in scale A (except for polymer 60P). Hardness in scale D was determined only for SPURs, PHCD derivatives, and the values for those polymers increased with increasing the content of the hard segment. In the case of carboxylated SPURs, hardness of the polymers obtained increased with the increase in the DMPA content (in scale A). When comparing hardness for carboxylated SPURs with PTMO and PHCD, it can be stated that greater values were shown by polymers containing polycarbonate soft segment and it was possible to mark the hardness in scale D for all those polymers as well.
The polymers obtained showed a tensile strength in the range of 0.42–35.2 MPa and their elongation at break ranged between 340 and 1900 %. SPURs, PTMO derivatives, showed very poor tensile strength. There are considerable greater strengths in the series of SPURs with polycarbonate soft segment, and the greatest tensile strength value was shown by the polymer 60C (35.2 MPa), whereas that polymer may fall into plastomers (T g slightly above room temperature). Both in PTMO and PHCD series, elongations at break decreased with the increase in the content of the hard segment (except for polymer 30P); however, no such relationship was observed for the module whose values ranged from 0.26 to 49.5 MPa.
Tensile strength of carboxylated SPURs increased with the increase in the content of DMPA. The polymers derived from PTMO showed significantly lower tensile strength values (0.92 and 1.24 MPa) than the corresponding ones with PHCD (12.1 and 29.4 MPa). Elongation at break of carboxylated SPURs, just as their tensile strength, increased with the increase in the content of DMPA, whereas that increase was more pronounced in the case of the polymers with PHCD as the soft segment. Significantly lower values of elongation at break were shown by the polymers synthesized with PHCD as the soft segment than in PTMO-based ones. For carboxylated SPURs with PTMO elastic modulus, values were in the range of 1.90 to 2.12 MPa, while for the polymers with PHCD they ranged between 1.02 and 5.92 MPa.
Conclusions
The polymers obtained were colourless, transparent solids with high molar masses. These polymers were characterized by relatively good thermal stability. Their T 5 values were in the temperature range of 285–320 °C, whereas polymers synthesized with PTMO were more stable thermally. The addition of DMPA to the polymers resulted in the decrease in their thermal stability.
DSC analyses showed that all polymers obtained were amorphous. Polymers with PTMO as the soft segment had significantly lower T g values (from −51 to −1 °C) than the corresponding ones with PHCD (from −10 to 29 °C). A comparison of T g of pure soft segments and the polymers obtained showed a much greater microphase separation ability of the polymers derived from PTMO. The hardness of the polymers obtained (in scale A) ranged from 19 to 81°Sh, whereas greater values corresponded to the polymers with PHCD. The best mechanical properties among all synthesized polymers were shown by the polymers with PHCD as the soft segment. Their tensile strength and elongation at break were 4.61–35.2 MPa and 340–900 %, respectively. The polymers with PTMO had much lower tensile strength values (0.42–1.24 MPa), but showed much greater elongation at break values at the same time (420–1900 %). A 40 mol% DMPA addition to the polymer in the series of carboxylated SPURs with PHCD resulted in a significant increase in strength.
References
Wirpsza Z. Polyurethanes: chemistry, technology and applications. New York: Ellis Horwood; 1993.
Ulrich H. Polyurethanes. In: Mark HF, editor. Encyclopedia of polymer science and technology, vol. 4. New Jersey: Wiley; 2003.
Gogolewski S. Selected topics in biomedical polyurethanes. A review. Colloid Polym Sci. 1989;267:757–85.
Zia KM, Bhatti IA, Barikani M, Zuber M, Shikh MA. Thermo-mechanical characteristics of UV-irradiated polyurethane elastomers extended with α, ω-alkane diols. Nucl Instrum Meth B. 2009;267:1811–6.
Kultys A, Rogulska M, Pikus S. New thermoplastic segmented polyurethanes with hard segment derived from 4,4′-diphenylmethane diisocyanate and methylenebis(1,4-phenylenemethylenethio)dialcanols. J Appl Polym Sci. 2012;123:331–46.
Kultys A, Rogulska M, Gluchowska H. The effect of soft segment structure on the properties of novel thermoplastic polyurethane elastomers based on an unconventional chain extender. Polym Int. 2011;60:652–9.
Kultys A, Rogulska M. New thermoplastic poly(carbonate-urethane) elastomers. Pol J Chem Technol. 2011;13:23–30.
Kultys A, Rogulska M, Pikus S, Skrzypiec K. The synthesis and characterization of new thermoplastic poly(carbonate-urethane) elastomers derived from HDI and aliphatic-aromatic chain extenders. Eur Polym J. 2009;45:2629–43.
Kultys A, Pikus S. Polyurethanes containing sulfur. III. New thermoplastic HDI-based segmented polyurethanes with diphenylmethane unit in their structure. J Polym Sci Pol Chem. 2001;39:1733–42.
Kultys A, Podkoscielny W, Majewski W. Polyurethanes containing sulfur. II. New thermoplastic nonsegmented polyurethanes with diphenylmethane unit in their structure. J Polym Sci Part A Polym Chem. 2000;38:1767–73.
Kultys A, Puszka A. New thermoplastic polyurethane elastomers based on sulfur-containing chain extenders. Pol J Chem Technol. 2013;15:65–70.
Kultys A, Puszka A. Transparent poly(thiourethane-urethane)s based on dithiol chain extender. J Therm Anal Calorim. 2014;117:1427–39.
Rogulska M, Kultys A, Olszewska E. New thermoplastic poly(thiourethane-urethane) elastomers based on hexane-1,6-diyl diisocyanate (HDI). J Therm Anal Calorim. 2013;114:903–16.
Rogulska M, Podkościelny W, Kultys A, Pikus S, Pozdzik E. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. I. Synthesis and characterization of nonsegmented polyurethanes from HDI and MDI. Eur Polym J. 2006;42:1786–97.
Rogulska M, Kultys A, Podkościelny W. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. II. Synthesis and characterization of segmented polyurethanes from HDI and MDI. Eur Polym J. 2007;43:1402–14.
Rogulska M, Kultys A, Pikus S. Studies on thermoplastic Polyurethanes based on new diphenylethane-derivative diols. III. The effect of molecular mass and structure of soft segment on some properties of segmented polyurethanes. J Appl Polym Sci. 2008;110:1677–89.
Kultys A, Podkościelny W, Pikus S. Polyurethanes containing sulfur. I. New thermoplastic polyurethanes with benzophenone unit in their structure. J Polym Sci Pol Chem. 1999;37:4140–50.
Kultys A. Polyesters containing sulfur. VIII. New benzophenone-derivative thiopolyesterdiols synthesis and characterization. Their use for thermoplastic polyurethanes preparation. J Polym Sci Pol Chem. 2000;38:3977–83.
Kultys A, Rogulska M, Pikus S. The synthesis and characterization of new thermoplastic poly(thiourethane-urethane)s. J Polym Sci Pol Chem. 2008;46:1770–82.
Wdowicka D, Podkoscielny W, Kultys A. Polyurethanes (III): polyaddition products of 4,4′-Bis(2-hydroxyethylenethio)diphenyl ether and 2,4-tolylene diisocyanate. Iran Polym J. 2000;9:97–103.
Rogulska M, Kultys A, Lubczak J. New thermoplastic polyurethane elastomers based on aliphatic-aromatic chain extenders with different content of sulfur atoms. J Therm Anal Calorim. 2015;121:397–410.
Rogulska M, Kultys A. Aliphatic polycarbonate-based thermoplastic polyurethane elastomers containing diphenyl sulfide units. J Therm Anal Calorim. 2016;. doi:10.1007/s10973-016-5420-z.
Kultys A. Sulfur-containing polymers. In: Mark HF, editor. Encyclopedia of polymer science and technology, vol. 4. New Jersey: Wiley; 2003. p. 336–93.
Hirano H, Kadota J, Agari Y, Harada T, Tanaka M, Hasegawa K. Linear polymers with sulfur in the main chain. IV. Synthesis of thermotropic liquid-crystalline polythioesters based on 4,4′-biphenyldithiol with excellent adhesive properties. Polym Eng Sci. 2007;47:262–9.
Čulin J, Šmit I, Veksli Z, Anžlovar A, Žigon M. Phase morphology of functionalized polyester polyurethanes. Effect of functional group concentration. Polym Int. 2006;55:285–91.
KosheelaDevi PP, Tuan Noor Maznee TI, Hoong SS, Nurul’Ain H, Mohd. Norhisham S, Norhayati MN, Srihanum A, Yeong SK, Hazimah AH, Sadijarevic V, Sandijarevic A. Performance of palm oil-based dihydroxystearic acid as ionizable molecule in waterborne polyurethane dispersions. J Appl Polym Sci. 2016;. doi:10.1002/app.43614.
Žagar E, Žigon E. Dilute solution behaviour of hexamethylene diisocyanate-based carboxylated polyurethanes and related ionomers in tetrahydrofuran. Polymer. 1999;40:2727–35.
Žagar E, Žigon E. Solution properties of carboxylated polyurethanes nad related ionomers in polar solvents (DMF and LiBr/DMF). Polymer. 2000;41:3513–21.
Zhang S, Miao W, Zhou Y. Reaction study of water-borne polyurethanes based on isophorone diisocyanate, dimethylol propionic acid, and poly(hexane neopentyl adipate glycol). J Appl Polym Sci. 2004;92:161–4.
Matsunaga K, Nakagawa K, Sawai S, Sonoda O, Tajima M, Yoshida Y. Synthesis and characterization of polyurethane anionomers. J Appl Polym Sci. 2005;98:2144–8.
Barikani M, Valipour Ebrahimi M, Seyed Mohaghegh SM. Preparation and characterization of aqueous polyurethane dispersions containing ionic centers. J Appl Polym Sci. 2007;104:3931–7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Puszka, A., Kultys, A. New thermoplastic polyurethane elastomers based on aliphatic diisocyanate. J Therm Anal Calorim 128, 407–416 (2017). https://doi.org/10.1007/s10973-016-5923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5923-7