Abstract
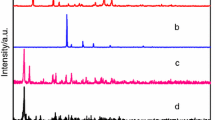
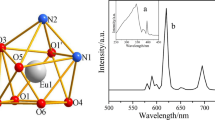
Two novel mononuclear lanthanide complexes, [Ln(2,4-DClBA)3(terpy)(H2O)]·H2O (Ln = Dy(1), Er(2)); 2,4-DClBA: 2,4-dichlorobenzoate; terpy: 2,2′:6′,2″-terpyridine; have been synthesized and characterized by single-crystal and powder X-ray diffraction. The results reveal that complexes 1–2 are isomorphous, and each Ln3+ ion is nine coordinated adopting a distorted monocapped square antiprismatic molecular geometry. Mononuclear complexes 1–2 are stitched together via Cl–π and hydrogen bonding interactions to form the 1D, 2D, 3D supramolecular structures, which the 3D is rarely observed. Thermal decomposition mechanism is determined by TG/DSC-FTIR, and the 3D stacked plots for the FTIR spectra of the evolved gases were recorded. Heat capacities of complexes 1–2 were measured, and the derived thermodynamic functions (H T − H 298.15), (S T − S 298.15) and (G T − G 298.15) of the complexes relative to the standard reference temperature 298.15 K were obtained. The activation energy E values of the first decomposition stage for title complexes were calculated by integral isoconversional nonlinear (NL-INT) and Starink methods. Complex 1 shows the characteristic emission of Dy3+ with strong yellow emission.









Similar content being viewed by others
References
Li RF, Liu XF, Zhang T, Wang LY, Ma LF, Feng X. Two unique lanthanide–organic frameworks based on biphenyl-2,3,3′,5′-tetracarboxylic acid: syntheses, crystal structures and luminescence properties. Polyhedron. 2015;99:238–43.
Zhang S, Duan E, Han Z, Li L, Cheng P. Lanthanide coordination polymers with 4,4′-azobenzoic acid: enhanced stability and magnetocaloric effect by removing guest solvents. Inorg Chem. 2015;54:6498–503.
Maity M, Majee MC, Kundu S, Samanta SK, Sanudo EC, Ghosh S, Chaudhury M. Pentanuclear 3d–4f heterometal complexes of M(II)3Ln(III)2 (M = Ni, Cu, Zn and Ln = Nd, Gd, Tb) combinations: syntheses, structures, magnetism, and photoluminescence properties. Inorg Chem. 2015;54:9715–26.
Ay B, Karaca S, Yildiz E, Lopez V, Nanao MH, Zubieta J. In situ hydrothermal syntheses, structures and photoluminescent properties of four novel metal-organic frameworks constructed by lanthanide (Ln = Ce(III), Pr(III), Eu(III)) and Cu(I) metals with flexible dicarboxylate acids and piperazine-based ligands. J Solid State Chem. 2016;233:415–21.
Sun X, Jin X, Pan W, Wang J. Syntheses of new rare earth complexes with carboxymethylated polysaccharides and evaluation of their in vitro antifungal activities. Carbohydr Polym. 2014;113:194–9.
Zong GC, Huo JX, Ren N, Zhang JJ, Qi XX, Gao J, Geng LN, Wang SP, Shi SK. Preparation, characterization and properties of four new trivalent lanthanide complexes constructed using 2-bromine-5-methoxybenzoic acid and 1,10-phenanthroline. Dalton Trans. 2015;44:14877–86.
Gu JZ, Wu J, Lv DY, Tang Y, Zhu K, Wu J. Lanthanide coordination polymers based on 5-(2′-carboxylphenyl) nicotinate: syntheses, structure diversity, dehydration/hydration, luminescence and magnetic properties. Dalton Trans. 2013;42:4822–30.
Yang L, Liu L, Wu L, Xu Z, Wang L. Isomorphous and isostructural lanthanide coordination polymers based on 2-(4-chlorobenzoyl)benzoic acid: synthesis, structure, characterization, and luminescent properties. Dyes Pigments. 2014;111:176–84.
Oliveira CK, de Souza VP, da Luz LL, de Menezes Vicenti JR, Burrow RA, Severino A, Longo RL, Malvestiti I. Synthesis, crystal structure and luminescent properties of lanthanide extended structure with asymmetrical dinuclear units based on 2-(methylthio)benzoic acid. J Lumin. 2016;170:528–37.
Du M, Wang X, Chen M, Li CP, Tian JY, Wang ZW, Liu CS. Ligand symmetry modulation for designing a mesoporous metal-organic framework: dual reactivity to transition and lanthanide metals for enhanced functionalization. Chemistry. 2015;21:9713–20.
Xu C, Kirillov AM, Shu Y, Liu Y, Guo L, Yang L, Dou W, Liu W, Chen C, Huang X, Zhang J, Liu W. Photoluminescence enhancement induced by a halide anion encapsulation in a series of novel lanthanide(iii) coordination polymers. CrystEngComm. 2016;18:1190–9.
Ay B, Yildiz E, Kani İ. Novel heteroleptic lanthanide organic frameworks containing pyridine-2,5-dicarboxylic acid and in situ generated piperazine-2,5-dicarboxylic acid from piperazine: hydrothermal synthesis and luminescent properties. J Solid State Chem. 2016;233:44–51.
Marques LF, Cuin A, de Carvalho GSG, dos Santos MV, Ribeiro SJL, Machado FC. Energy transfer process in highly photoluminescent binuclear hydrocinnamate of europium, terbium and gadolinium containing 1,10-phenanthroline as ancillary ligand. Inorg Chim Acta. 2016;441:67–77.
Marques LF, Cantaruti AAB, Correa CC, Lahoud MG, da Silva RR, Ribeiro SJL, Machado FC. First crystal structures of lanthanide-hydrocinnamate complexes: hydrothermal synthesis and photophysical studies. J Photochem Photobiol A Chem. 2013;252:69–76.
Carter KP, Pope SJA, Cahill CL. A series of Ln-p-chlorobenzoic acid–terpyridine complexes: lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm. 2014;16:1873.
Zhang YY, Ren N, Xu SL, Zhang JJ, Zhang DH. A series of binuclear lanthanide(III) complexes: crystallography, antimicrobial activity and thermochemistry properties studies. J Mol Strut. 2015;1081:413–25.
Zhang YY, Ren SH, Ren N, Zhang JJ, Geng LN, Wang SP, Shi SK. Crystal structures, spectroscopic, and thermal properties of dysprosium(III) and europium(III) complexes with 3-chloro-4-methoxybenzoic and 1,10-phenanthroline. J Therm Anal Calorim. 2015;119:1803–10.
Qi XX, Wu JC, Ren N, Zhao CL, Zhang JJ, Zong GC, Gao J. Novel lanthanide complexes constructed from 3, 4-dimethoxybenzoic acid: crystal structures, spectrum and thermochemical properties. Thermochim Acta. 2015;615:1–7.
Wang MH, Tan ZC, Sun XH, Zhang HT, Liu BP, Sun LX, Zhang T. Determination of heat capacities and thermodynamic properties of 2-(chloromethylthio)benzothiazole by an adiabatic calorimeter. Chem Eng Data. 2005;50:270–3.
Liu JY, Ren N, Zhang JJ, He SM, Wang SP. Crystal structures, thermal properties, and biological activities of a series of lanthanide compounds with 2,4-dichlorobenzoic acid and 1,10-phenanthroline. Ind Eng Chem Res. 2013;52:6156–63.
Huo JX, Wang Y, Zhang DH, Ren N, Zhang JJ. Syntheses, characterization, luminescence, and thermal decomposition mechanism of four lanthanide complexes with 4-ethylbenzoic acid and terpyridine. J Therm Anal Calorim. 2016;124:1575–85.
Carter KP, Zulato CHF, Cahill CL. Exploring supramolecular assembly and luminescent behavior in a series of RE-p-chlorobenzoic acid-1,10-phenanthroline complexes. CrystEngComm. 2014;16:10189–202.
Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Efficient catalytic addition of aromatic carbon–hydrogen bonds to olefins. Nature. 1993;366:529–31.
Zhou JM, Shi W, Xu N, Cheng P. Highly selective luminescent sensing of fluoride and organic small-molecule pollutants based on novel lanthanide metal-organic frameworks. Inorg Chem. 2013;52:8082–90.
Onoda M, Yamamoto A, Takayama-Muromachi E, Takekawa S. Assignment of the powder X-ray diffraction pattern of superconductor Bi2(Sr,Ca)3–xCu2Oy. Jpn J Appl Phys. 1988;27:L833–6.
Ye HM, Ren N, Zhang JJ, Sun SJ, Wang JF. Crystal structures, luminescent and thermal properties of a new series of lanthanide complexes with 4-ethylbenzoic acid. New J Chem. 2010;34:533.
Vyazovkin S, Dollimore D. Linear and nonlinear procedures in isoconversional computations of the activation energy of nonisothermal reactions in solids. J Chem Inf Mode. 1996;36:42–5.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Therm Acta. 2003;404:163–76.
Vyazovkin S. Kinetic concepts of thermally stimulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Qi XX, Ren N, Zhang DH, Zhang JJ. Synthesis, spectroscopic, thermochemical properties of lanthanide complexes with 3,4-diethoxybenzoic acid and 1,10-phenanthroline. Chem Res Chin Univ. 2015;31:1039–45.
Jin CW, Wang Y, Xu SL, Zhang JJ. Synthesis, crystal structures and thermochemical properties of ternary rare earth complexes based on 3,4-diethoxybenzoic acid and 2,2′-bipyridine. Acta Phys Chim Sin. 2016. doi:10.3866/PKU.WHXB201605263.
Acknowledgements
The research work was supported by the National Natura Science Foundation of China (No. 21473049) and the Natural Science Foundation of Hebei Province (No. B2016205207).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhao, QQ., Ren, N. et al. Crystal structures, thermal properties, and luminescent properties of two novel mononuclear lanthanide complexes with 2,4-dichlorobenzoic acid and 2,2′:6′,2″-terpyridine. J Therm Anal Calorim 126, 1703–1712 (2016). https://doi.org/10.1007/s10973-016-5728-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5728-8