Abstract
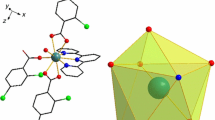
Three novel ternary lanthanide complexes [Ln(2,5-DClBA)3(terpy)(H2O)] (Ln = Eu (1), Tb (2), Ho (3), 2,5-DClBA = 2,5-dichlorobenzoate, terpy = 2,2:6′,2″-terpyridine) have been successfully synthesized and characterized by single-crystal and powder X-ray diffraction. Complexes 1–3 are isostructural, and each metal center is nine-coordinated with distorted monocapped square antiprismatic coordination geometry. The mononuclear units are assembled into 1D, 2D, 3D supramolecular structures by the π–π stacking and halogen–halogen interactions. Thermal decomposition mechanisms of the title complexes were investigated by thermogravimetric analysis and further authenticated by TG/DSC-FTIR techniques. The solid-state fluorescent properties of complexes 1–2 were studied at room temperature, and characteristic emission was observed for all complexes. Complex 1 exhibits the strong red emission, while the complex 2 is green under UV light. Moreover, the antibacterial activities of the complexes against Candida albicans, Escherichia coli and Staphylococcus aureus were also evaluated.










Similar content being viewed by others
References
Carter KP, Thomas KE, Pope SJ, Holmberg RJ, Butcher RJ, Murugesu M. Supramolecular assembly of molecular rare-earth-3,5-dichlorobenzoic acid-2,2′:6′,2″-terpyridine materials: structural systematics, luminescence properties, and magnetic behavior. Inorg Chem. 2016;55(14):6902–15. doi:10.1021/acs.inorgchem.6b00408.
Zong GC, Huo JX, Ren N, Zhang JJ, Qi XX, Gao J. Preparation, characterization and properties of four new trivalent lanthanide complexes constructed using 2-bromine-5-methoxybenzoic acid and 1,10-phenanthroline. Dalton Trans. 2015;44(33):14877–86. doi:10.1039/c5dt01969a.
Faulkner S, Pope SJA, Burton-Pye BP. Lanthanide complexes for luminescence imaging applications. Appl Spectrosc Rev. 2005;40(1):1–31. doi:10.1081/asr-200038308.
Lhoste J, Perez-Campos A, Henry N, Loiseau T, Rabu P, Abraham F. Chain-like and dinuclear coordination polymers in lanthanide (Nd, Eu) oxochloride complexes with 2,2′:6′,2″-terpyridine: synthesis, XRD structure and magnetic properties. Dalton Trans. 2011;40(36):9136–44. doi:10.1039/c1dt10485c.
Guo X, Zhu G, Fang Q, Xue M, Tian G, Sun J. Synthesis, structure and luminescent properties of rare earth coordination polymers constructed from paddle-wheel building blocks. Inorg Chem. 2005;44(11):3850–5.
Wei XH, Yang LY, Liao SY, Zhang M, Tian JL, Du PY. A series of rare earth complexes with novel non-interpenetrating 3D networks: synthesis, structures, magnetic and optical properties. Dalton Trans. 2014;43(15):5793–800. doi:10.1039/c3dt53112k.
Kelly NR, Goetz S, Batten SR, Kruger PE. Coordination behaviour and network formation with 4,4′,6,6′-tetracarboxy-2,2′-bipyridine and 4,4′-dicarboxy-2,2′-bipyridineligands with rare and alkaline earth metals. CrystEngComm. 2008;10(1):68–78. doi:10.1039/b711469a.
Zhao Y, Shi C, Yang X, Shen B, Sun Y, Chen Y. pH- and temperature-sensitive hydrogel nanoparticles with dual photoluminescence for bioprobes. ACS Nano. 2016;10(6):5856–63. doi:10.1021/acsnano.6b00770.
Bünzli J-CG. Lanthanide luminescent bioprobes (LLBs). Acs Med Chem Lett. 2009;38(2):104–9. doi:10.1246/cl.2009.104.
Gribkov DV, Hultzsch KC, Hampel F. Synthesis and characterization of new biphenolate and binaphtholate rare-Earth-metal amido complexes: catalysts for asymmetric olefin hydroamination/cyclization. Chemistry. 2003;9(19):4796–810. doi:10.1002/chem.200304975.
Zhan CH, Wang F, Kang Y, Zhang J. Lanthanide-thiophene-2,5-dicarboxylate frameworks: ionothermal synthesis, helical structures, photoluminescent properties, and single-crystal-to-single-crystal guest exchange. Inorg Chem. 2012;51(1):523–30. doi:10.1021/ic201986m.
Marques LF, Santos HP, Correa CC, Resende JALC, da Silva RR, Ribeiro SJL. Construction of a series of rare earth metal-organic frameworks supported by thiophenedicarboxylate linker: synthesis, characterization, crystal structures and near-infrared/visible luminescence. Inorg Chim Acta. 2016;451:41–51. doi:10.1016/j.ica.2016.07.008.
Einkauf JD, Kelley TT, Chan BC, de Lill DT. Rethinking sensitized luminescence in lanthanide coordination polymers and MOFs: band sensitization and water enhanced eu luminescence in [Ln(C15H9O5)3(H2O)3]n (Ln = Eu, Tb). Inorg Chem. 2016;55(16):7920–7. doi:10.1021/acs.inorgchem.6b00878.
Lahoud MG, Muniz EC, Arroyos G, Fávaro MA, Davolos MR, D’Vries RF. Rare earth coordination dinuclear compounds constructed from 3,5-dicarboxypyrazolate and succinate intermetallic bridges. New J Chem. 2016;40(6):5338–46. doi:10.1039/c6nj00140h.
Hasegawa Y, Wada Y, Yanagida S. Strategies for the design of luminescent lanthanide(III) complexes and their photonic applications. J Photochem Photobiol C. 2004;5(3):183–202. doi:10.1016/j.jphotochemrev.2004.10.003.
Eliseeva SV, Bunzli JC. Lanthanide luminescence for functional materials and bio-sciences. Chem Soc Rev. 2010;39(1):189–227. doi:10.1039/b905604c.
Bünzli J-CG, Comby S, Chauvin A-S, Vandevyver CDB. New opportunities for lanthanide luminescence. J Rare Earth. 2007;25(3):257–74. doi:10.1016/s1002-0721(07)60420-7.
De Sa G, Malta O, de Mello Donegá C, Simas A, Longo R, Santa-Cruz P. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coordin Chem Rev. 2000;196(1):165–95.
Liu L, Xu Z, Lou Z, Zhang F, Sun B, Pei J. Luminescent properties of a novel terbium complex Tb(o-BBA)3(phen). J Rare Earth. 2006;24(2):253–6. doi:10.1016/s1002-0721(06)60104-x.
Zong G-C, Ren N, Zhang J-J, Qi X-X, Gao J. Lanthanide complexes with 3-bromine-4-methyl benzoic acid and 1,10-phenanthroline. J Therm Anal Calorim. 2015;123(1):105–16. doi:10.1007/s10973-015-4901-9.
Qi X-X, Ren N, Xu S-L, Zhang J-J, Zong G-C, Gao J. Lanthanide complexes with 3,4,5-triethoxybenzoic acid and 1,10-phenanthroline: synthesis, crystal structures, thermal decomposition mechanism and phase transformation kinetics. RSC Adv. 2015;5(12):9261–71. doi:10.1039/c4ra12063a.
Koroteev PS, Dobrokhotova ZV, Ilyukhin AB, Efimov NN, Kirdyankin DI, Tyurin AV. Lanthanide cymantrenecarboxylate complexes with an Ln: Mn ratio of 1:2 as precursors for LnMn2O5 phases. Synthesis, structure, physicochemical properties, and thermal decomposition. Polyhedron. 2013;65:110–21. doi:10.1016/j.poly.2013.08.024.
Zheng J-R, Ren N, Zhang J-J, Zhang D-H, Yan L-Z, Wu K-Z. Synthesis, characterization and thermochemical properties of four new lanthanide complexes with 3,5-diisopropylsalicylic acid and 1,10-phenanthroline. Therm Acta. 2012;547:31–7. doi:10.1016/j.tca.2012.08.005.
Carter KP, Zulato CHF, Cahill CL. Exploring supramolecular assembly and luminescent behavior in a series of RE-p-chlorobenzoic acid-1,10-phenanthroline complexes. CrystEngComm. 2014;16(44):10189–202. doi:10.1039/c4ce01806k.
Matthes PR, Nitsch J, Kuzmanoski A, Feldmann C, Steffen A, Marder TB. The series of rare earth complexes [Ln2Cl6 (mu-4,4′-bipy)(py)6], Ln = Y, Pr, Nd, Sm-Yb: a molecular model system for luminescence properties in MOFs based on LnCl3 and 4,4′-bipyridine. Chemistry. 2013;19(51):17369–78. doi:10.1002/chem.201302504.
Carter KP, Pope SJA, Cahill CL. A series of Ln-p-chlorobenzoic acid–terpyridine complexes: lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm. 2014;16(10):1873. doi:10.1039/c3ce42267d.
Ahmed Z, Iftikhar K. Sensitization of visible and NIR emitting lanthanide(III) ions in noncentrosymmetric complexes of hexafluoroacetylacetone and unsubstituted monodentate pyrazole. J Phys Chem A. 2013;117(44):11183–201. doi:10.1021/jp403668j.
Feng R, Jiang F-L, Wu M-Y, Chen L, Yan C-F, Hong M-C. Structures and photoluminescent properties of the lanthanide coordination complexes with hydroxyquinoline carboxylate ligands. Cryst Growth Des. 2010;10(5):2306–13. doi:10.1021/cg100026d.
Sabbatini N, Guardigli M, Manet I. Antenna effect in encapsulation complexes of lanthanide ions. Handb Phys Chem Rare Earths. 1996;23:69–119.
Kirby AF, Richardson F. Detailed analysis of the optical absorption and emission spectra of europium (3 +) in the trigonal (C3) Eu (DBM) 3. H2O system. J Phys Chem. 1983;87(14):2544–56.
Rodrigues MO, da Costa Junior NB, de Simone CA, Araujo AA, Brito-Silva A, Paz FAA. Theoretical and experimental studies of the photoluminescent properties of the coordination polymer [Eu (DPA)(HDPA)(H2O) 2] ⊙ 4H2O. J Phys Chem B. 2008;112(14):4204–12.
Wang Y, Shen P-P, Ren N, Zhang J-J, Geng L-N, Wang S-P. A series of lanthanide complexes with different N-donor ligands: synthesis, structures, thermal properties and luminescence behaviors. RSC Adv. 2016;6(75):70770–80. doi:10.1039/c6ra11393a.
Zhao B, Chen X-Y, Cheng P, Liao D-Z, Yan S-P, Jiang Z-H. Coordination polymers containing 1D channels as selective luminescent probes. JACS. 2004;126(47):15394–5.
Huang J, Xu Y, Chen X, Xu D, Xu Y, He Q. Synthesis, characterization and properties of some rare earth complexes with 2,6-pyridine dicarboxylic acid and α-Picolinic acid. J Rare Earth. 2012;30(6):586–91. doi:10.1016/s1002-0721(12)60095-7.
Song YM, Xu JP, Ding L, Hou Q, Liu JW, Zhu ZL. Syntheses, characterization and biological activities of rare earth metal complexes with curcumin and 1,10-phenanthroline-5,6-dione. J Inorg Biochem. 2009;103(3):396–400. doi:10.1016/j.jinorgbio.2008.12.001.
Chaudhary A, Bansal N, Gajraj A, Singh RV. Antifertility, antibacterial, antifungal and percent disease incidence aspects of macrocyclic complexes of manganese(II). J Inorg Biochem. 2003;96(2–3):393–400. doi:10.1016/s0162-0134(03)00157-0.
Acknowledgements
The research work was supported by the National Natural Science Foundation of China (No. 21473049) and the Natural Science Foundation of Hebei Province (No. B2016205207).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhao, QQ., Wu, XH., Ren, N. et al. Synthesis, crystal structures, luminescence and thermal properties of lanthanide complexes containing 2,5-dichlorobenzoic acid and 2,2:6′,2″-terpyridine. J Therm Anal Calorim 131, 1237–1248 (2018). https://doi.org/10.1007/s10973-017-6591-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6591-y