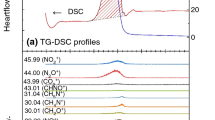
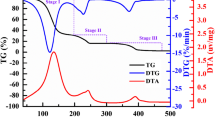
Abstract
Kinetics analyses were performed on the thermal decomposition of ammonium dinitramide (ADN) using thermogravimetry-differential thermal analysis–mass spectrometry–infrared spectroscopy (TG-DTA–MS–IR). The main evolved gases were determined to be NH3, H2O, N2, NO, N2O, and NO2. The apparent activation energies of the exothermic, mass-change and gas-evolving reactions were analyzed on the basis of Friedman methods. The apparent activation energy of evolving N2 has the same value as that of evolving H2O since they occur by the same mechanism. A Friedman plot obtained from the DTA data has a curve similar to those obtained from N2 and H2O. The reaction that generated N2 and H2O plays an important role in the exothermic reaction in the decomposition of ADN. The activation energy for the N2O evolution reaction has a range of approximately 120–152 kJ mol−1 with reaction progress values between 0.1 and 0.9. Quantum chemistry calculations revealed that the total energy barrier of dinitramic acid unimolecular decomposition and ammonium-dinitramic ions collision-induced decomposition is 149.9–156.0 and 160.6 kJ mol−1, respectively. These values are reasonable compared with the experimental value of 152 kJ mol−1.






Similar content being viewed by others
References
Larsson A, Wingborg N. Green propellants based on ammonium dinitramide (ADN). In: Hall J editor. Advances in spacecraft technologies. InTech; 2011. pp. 139–156.
Nagamachi MY, Oliveira JI, Kawamoto AM, Dutra RCL. ADN: The new oxidizer aroudthe corner for an environmentally friendly smokeless propellant. J Aerosp Technol Manag. 2009;1:153–60.
Östmark H, Bemm U, Langlet A, Sanden R, Wingborg N. The properties of ammonium dinitramide (ADN): part 1, basic properties and spectroscopic data. J Energ Mater. 2000;18:123–8.
Thakre P, Duan Y, Yang V. Modeling of ammonium dinitramide (ADN) monopropellant combustion with coupled condensed and gas phase kinetics. Combust Flame. 2014;161:347–62.
Sinditskii VP, Egorshev VY, Levshenkov AI, Serushkin VV. Ammonium nitrate: combustion mechanism and the role of additives. Propellants Explos Pyrotech. 2005;30:269–80.
Sinditskii VP, Egorshev VY, Serushkin VV, Filatov SA. Combustion of energetic materials controlled by condensed-phase reactions. Combust Explos Shock. 2012;48:81–99.
Ermolin NE. Modeling of pyrolysis of ammonium dinitramide sublimation products under low-pressure conditions. Combust Explos Shock. 2004;40:92–100.
Park J, Chakraborty D, Lin MC. Thermal decomposition of gaseous ammonium dinitramide at low pressure: kinetic modeling of product formation with ab initio Mo/cVRRKM calculations. Twenty-seventh symposium (international) on combustion/The Combustion Institute. 1998. pp. 2351–2357.
Raman S, Ashcraft RW, Vial M, Klasky ML. Oxidation of hydroxylamine by nitrous and nitric acids. Model development from first principle SCRF calculation. J Phys Chem A. 2005;109:8526–36.
Ashcraft RW, Raman S, Green WH. Ab initio aqueous thermochemistry: application to the oxidation of hydroxylamine in nitric acid solution. J Phys Chem B. 2007;111:11968–83.
Ashcraft RW, Raman S, Green WH. Predicted reaction rates of HxNyOz intermediates in the oxidation of hydroxylamine by aqeous nitric acid. J Phys Chem A. 2008;112:7577–93.
Kumbhakarna NR, Shah KJ, Chowdhury A, Thynell ST. Identification of liquid-phase decomposition species and reactions for guanidinium azotetrazolate. Thermochim Acta. 2014;590:51–65.
Kumbhakarna N, Thynell ST. Development of a reaction mechanism for liquid-phase decomposition of guanidinium 5-amino tetrazolate. Thermochim Acta. 2014;582:25–34.
Tompa AS. Thermal analysis of ammonium dinitramide (ADN). Thermochim Acta. 1999;357–358:177–93.
Oxley JC, Smith JL, Zhang W. Thermal decomposition studies on ammonium dinitramide (ADN) and 15N and 2H isotopomers. J Phys Chem. 1997;101:5646–52.
Vyazovkin S, Wight C. Ammonium dinitramide: kinetics and mechanism of thermal decomposition. J Phys Chem. 1997;101:5653–8.
Matsunaga H, Habu H, Miyake A. Thermal behavior of new oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;111:1183–8.
Yang R, Thakre P, Yang V. Thermal decomposition and combustion of ammonium dinitramide (review). Combust Explos Shock. 2005;41:657–79.
Langlet A, Wingborg N, Östmart H. ADN: a new and promising oxidizer for composite rocket propellants. In: Kuo K editor. Challenges in propellants and combustion: 100 years after Nobel. Begell House; 1997. pp.616–626.
Matsunaga H, Habu H, Miyake A. Thermal decomposition of the high-performance oxidizer ammonium dinitramide under pressure. J Therm Anal Calorim. 2014;116:1227–32.
Matsunaga H, Izato Y, Habu H, Miyake A. Thermal decomposition characteristics of mixtures of ammonium dinitramide and copper(II) oxide. J Therm Anal Calorim. 2015;121:319–26.
Chai JD, Head-Gordon M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys. 2008;10:6615–20.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford CT; 2010.
Montgomery JA, Frisch MJ, Ochterski JW, Petersson GA. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys. 1999;110:2822–7.
Matsunaga H, Habu H, Miyake A. Influences of aging on thermal decomposition mechanism of high performance oxidizer ammonium dinitramide. J Therm Anal Calorim. 2013;113:1387–94.
Linstrom PJ, Mallard WG. NIST Chemistry WebBook. NIST standard reference database number 69. Eds. National Institute of Standards and Technology. http://webbook.nist.gov. Accessed 30 May 30 2016.
Vyazovkin S, Wight C. Thermal decomposition of ammonium dinitramide at moderate and high temperatures. J Phys Chem. 1997;101:7217–21.
Russell TP, Stern AG, Koppes WM, Bedford CD. Thermal decomposition and stabilization of ammonium dinitramide. Proc. 29th JANNAF combustion subcommittee meeting. CPIA Publ. 1992;593:339–345.
Korobeinichev OP, Kuibida LV, Paletsky AA, Shmakov AG. Molecular-beam mass-spectrometry to ammonium dinitramide combustion chemistry studies. J Propuls Power. 1998;14:991–1000.
Rossi MJ, Bottaro JC, McMillen DF. The thermal decomposition of the new energetic material ammonium dinitramide [NH4N(NO2)2] in relation to nitramide (NH2NO2) and NH4NO3. Int J Chem Kinet. 1993;25:549–70.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1963;6:183–95.
Ozawa T. Applicability of Friedman plot. J Therm Anal. 1986;31:547–51.
Lord FM, Kittelberger JS. On the determination of activation energies in thermal desorption experiments. Surf Sci. 1974;43:173–82.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Kazakov AI, Rubtsov YI, Manelis GB. Kinetics of the thermal decomposition of dinitramide 2. Kinetics of the reactions of dinitramide with decomposition products and other components of a solution. Russ Chem Bull. 1998;47:39–45.
Rosser WA, Inami SH, Wise H. The kinetics of decomposition of liquid ammonium nitrate. J Phys Chem. 1963;67:1753–7.
Izato Y, Miyake A. A condensed phase decomposition mechanism for ammonium nitrate. Sci Technol Energ Mater. 2015;76:98–103.
Oxley JC, Smith JL, Wang W. Compatibility of ammonium nitrate with monomolecular explosives. Part II Nitroarenes. J Phys Chem. 1994;98:3901–7.
Chaturvedi S, Dave PN. Review on thermal decomposition of ammonium nitrate. J Energ Mater. 2012;31:1–26.
Keenan AG, Notz K, Franco NB. Synergistic catalysis of ammonium nitrate decomposition 1. J Am Chem Soc. 1969;91:3168–71.
Izato Y, Miyake A. Thermal decomposition mechanism of ammonium nitrate and potassium chloride mixture. J Therm Anal Calorim. 2015;121:287–94.
Krautle KJ, Atwood AJ. The reaction of ammonium dinitramide under thermal load. Proc. 29th JANNAF Combust. Subcommittee Meeting. CPIA Publ. 1992. pp. 593:157.
Rahm M, Brinck T. Dinitraminic acid (HDN) isomerization and self-decomposition revisited. Chem Phys. 2008;348:53–60.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Izato, Yi., Koshi, M., Miyake, A. et al. Kinetics analysis of thermal decomposition of ammonium dinitramide (ADN). J Therm Anal Calorim 127, 255–264 (2017). https://doi.org/10.1007/s10973-016-5703-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5703-4