Abstract
The synthesis and thermal and optical properties of a new type of photoluminescent monomer of 2,7-bis[2-(2-hydroxy-3-methacryloyloxypropoxy)propoxy]naphthalene (2,7-NAF.PMEM) are presented. The chemical structure of 2,7-NAF.PMEM was confirmed by 1H and 13C NMR analysis and ATR-FTIR spectroscopy. Next, bulk copolymers were synthesized using two comonomers, methyl methacrylate and styrene. These monomers were chosen because of their good optical properties (high visible light transparency). The thermal properties of the new copolymers were studied by the TG/DSC/QMS-coupled method in an inert atmosphere. The UV–Vis absorption and luminescence spectra of the obtained polymers were registered and evaluated. The 510-nm luminescence band was observed independently of the kind of monomer and its concentration.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been visible progress in the development of polymeric substances showing the ability of luminescence. The photoluminescent materials used so far are being replaced with much cheaper organic compounds which do not emit harmful radiation, particularly hydrocarbon derivatives of naphthalene. These compounds with unique luminescent properties are investigated and used as fluorophores [1, 2], polymer light-emitting diodes [3, 4], and luminophores in optical devices [5–7].
Polymers based on naphthalene derivatives also have interesting luminescent properties. The influence of the structure on their macroscopic and electrooptical properties is very complicated and has not been fully elucidated. Naphthalene has extensive substitution chemistry, which allows it to be polymerized in different ways.
Π-conjugated polymers of naphthalene are potential candidates for the construction of electrodevices such as active layers in organic field-effect transistors (OFET) or distributed feedback polymer lasers (DFB) [3, 6–14]. Photoluminescence of naphthalene copolymers results from the excitation of π electrons in the UV–Vis region. Most frequently, green or yellow-green emission is observed. The value of the Stokes shift has been found to change depending on the kind of substituent in naphthalene structure, but no clear rules governing the magnitude of the Stokes shift have been discovered to date. Generally, the redshift observed in the luminescence spectra of naphthalene copolymers, when solid state is compared with liquid one, is rationalized as π–π interaction between polymer backbones.
On account of the unique photoluminescence properties of the dimethacrylate derivative of naphthalene-2,7-diol (2,7-NAF.DM), prospective studies of other naphthalene-2,7-diol derivatives seem to be interesting and promising [15–18]. Photoluminescence in 2,7-NAF.DM is undoubtedly connected with the arrangement of the substituents (2,7) in the naphthalene ring. Differently than substances which exhibit luminescence but do not possess vinyl bonds, naphthalate-2,7-diol derivatives enable the use of a new group of compounds in the area of optical polymers, thus broadening their potential specific and unique application. The proposed monomers can bind with other monomers by chemical bonds yielding products with better mechanical, chemical, and thermal properties. Additionally, obtained copolymers occur in a variety of forms (powders, blocks, straps, foils).
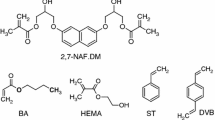
In the present study, methyl methacrylate (MMA), which is commonly used in the preparation of optical polymers, was used as a comonomer. Poly(methyl methacrylate) (PMMA) is largely transparent in the range of visible light, and its polymers are thermoplastic materials. Owing to these features, PMMA is used as the main optical material in the production of polymer optical fibers. A second monomer that was used in the polymerization reaction was styrene (St), the precursor of polystyrene.
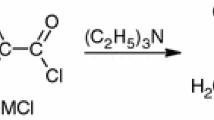
This work presents the synthesis of new dimethacrylate derivatives of naphthalene-2,7-diol. The 2,7-NAF.PMEM monomer was obtained as follows: firstly, naphthalene-2,7-diol was transformed into a dihydroxypropoxyl derivative of naphthalene-2,7-diol in a reaction with propylene carbonate at 210–220 °C in the presence of a catalyst. Then, the derivative obtained was subjected to a reaction with epichlorohydrin followed by a reaction with methacrylic acid, in the presence of TEBACl (triethylbenzylchloride) as a catalyst. A diester of the methacrylic acid derivative of naphthalene-2,7-diol (2,7-NAF.PMDM) was the final product. The chemical structures of all new compounds were confirmed by spectroscopic methods. Next, copolymerization reactions of 2,7-NAF.PMEM with different amounts of MMA or St were prepared. The thermal and luminescent properties of the new copolymers were studied.
Experimental
Synthesis of dimethacrylate derivatives of naphthalene-2,7-diol
Chemicals and eluents
Naphthalene-2,7-diol and 2-methacrylic acid were from Fluka AG (Buchs Switzerland). Dibenzoyl peroxide was obtained from Merck (Darmstadt, Germany). Reagent-grade propylene carbonate, potassium carbonate, chloroform, toluene, anhydrous potassium acetate, sodium hydroxide, toluene, xylene, sodium chloride, 2-(chloromethyl)oxirane, and hydroquinone were obtained from POCh (Gliwice, Poland). Triethylbenzylammonium chloride (TEBACl) was prepared in the laboratory of the Department of Polymer Chemistry, UMCS (Lublin, Poland). All above-mentioned chemicals were used as received.
Synthesis of 2,7-NAF.P-OH
In total, 43 g of naphthalene-2,7-diol, 55.1 g of propylene carbonate, and 0.13 g of potassium carbonate (as a catalyst) were placed in a 250-cm3 round-bottomed four-necked flask equipped with a mechanical stirrer, a thermometer, a reflux condenser and a glass tube (nitrogen atmosphere). During the reaction, CO2 was evolved for about 2.5 h, and the reaction mixture was heated in the range of 210–220 °C.
Subsequently, 200 cm3 of chloroform and 150 cm3 of distilled water were added to the flask. The flask contents were transferred to a separator funnel, and the organic phase was separated. After distilling of the organic solvent, 74 g of 2,7-bis(2-hydroxypropoxy)naphthalene (2,7-NAF.P-OH) was obtained. 2,7-NAF.P-OH was crystallized from toluene giving cream-colored crystals, mp = 73 °C.
Synthesis of 2,7-NAF.P-EP
In total, 51 g of 2,7-bis(2-hydroxypropoxy)naphthalene, 150 cm3 of 2-(chloromethyl)oxirane, and 0.4 g anhydrous potassium acetate (catalyst) were placed in a 500-cm3 round-bottomed three-necked flask equipped with a mechanical stirrer, a thermometer, and an azeotropic cap and stirred for 0.5 h. Next, 18.5 g of NaOH in 70 cm3 of xylene was added during 1 h. Finally, 400 cm3 of toluene was added and stirred for 15 min. When the reaction was over, the content of the flask was placed in a dropper. The water layer was separated along with the sodium chloride it contained, and the organic layer was distilled at a low pressure in order to remove solvents and 2-(chloromethyl)oxirane excess. The product, 2,7-NAF.P-EP was a high-viscosity liquid, L EP = 0.32 (L EP, epoxide number; L EP (Theor) = 0.51).
Synthesis of 2,7-NAF.PMEM
The synthesis was carried out in a 100-cm3 round-bottomed flask equipped with a thermometer, a mechanical stirrer, and a heater. To the flask were added 50 g of 2,7-NAF.P-EP, 13.7 g of methacrylic acid, triethylbenzylammonium chloride TEBACl (a catalyst), and hydroquinone (polymerization inhibitor). The reaction between the epoxide groups of 2,7-NAF.P-EP and the carboxylic groups of methacrylic acid was carried out for 4 h at 90 °C, and then for 3 h at 100 °C. The progress of the reaction was controlled by determination of the acid number. The reaction was considered to be over when the acid number was below 5. The chemical structure of the new monomer (2,7-NAF.PMEM) was confirmed by spectroscopic methods.
Copolymerization
Compositions consisting of 2,7-NAF.PMEM, one of the vinyl monomers (St or MMA), and an initiator (dibenzoyl peroxide, BPO) were prepared. A constant concentration of the initiator (1 %) and variable mass ratios of 2,7-NAF.PMEM to vinyl monomers (1:5 or 1:20) were applied. Next, the mixtures containing 2,7-NAF.PMEM, one of the vinyl monomers, and the initiator were placed together in a glass form, heated at 60 °C in a water bath for 12 h, and then transferred to a heater chamber and heated at 80 °C for 4 h.
Characterization of the products
ATR-FTIR spectra were obtained on a Bruker FTIR spectrophotometer TENSOR 27. TG/DTG/DSC analysis was carried out on a STA 449 Jupiter F1 instrument from Netzsch (Germany). Sample of about 10 mg was placed in Al2O3 crucibles and analyzed in flowing helium (40 cm3 min−1) at the heating rate of 10 °C min−1. Based on TG/DTG/DSC curves, the peak maximum decomposition temperature (T max), temperature of 5 % mass loss (T 5%), the final decomposition temperature (T f), and DSC maximum temperatures (T peak) were determined for each step.
Evolved gases analysis (EGA) was performed using a QMS 403 C Aëolos spectrometer (Germany). The QMS measurements were performed in the scan mode for m/z ranging from 10 to 120 amu.
Room-temperature UV–Vis reflectance spectra were registered using a Horizontal Sampling Integrating Sphere (Model PIV-756) connected to a V-660 JASCO spectrophotometer. Emission and excitation spectra were obtained with a Photon Technology International Inc. spectrofluorimeter equipped with a continuous wave Xe-arc lamp as a light source. Spectral resolution was maintained at 1 nm. Solid samples were measured in quartz cuvettes or in appropriate adapters.
Results and discussion
The scheme of reactions leading to the synthesis of the monomer 2,7-NAF.PMEM is shown in Fig. 1. The chemical structures of the monomers used in the copolymerization reaction are shown in Fig. 2. The copolymers obtained in the reaction contained aromatic or aliphatic fragments originating from St or MMA, respectively.
ATR-FTIR analysis
In order to confirm the course of the reaction at all stages, ATR-FTIR analyses were performed. The ATR-FTIR spectra for monomer 2,7-NAF.PMEM and its precursors 2,7-NAF.P and 2,7-NAF.P-EP are presented in Fig. 3. Absorption of aromatic naphthalene (C–H) was observed for all copolymers in the range of 830–834 cm−1.
A strong band at 3320 cm−1 was caused by stretching vibrations of O–H bonds in 2,7-NAF.P-OH, which confirmed the presence of hydroxypropylene fragments in its structure. In the spectra for 2,7-NAF.P-EP, there was a signal at 906 cm−1 corresponding to epoxy groups. The spectra for 2,7-NAF.PMEM contained a C=O signal at 1712 cm−1 coming from carboxylic groups.
NMR analysis
The chemical structures of 2,7-NAF.P-OH, 2,7-NAF.P-EP, and 2,7-NAF.P-PMEP were confirmed by 1H and 13C-NMR analysis. Detailed information on peaks is presented below. Additionally, 13C-NMR spectra for all the derivatives are shown in Fig. 4.
1 H-NMR
(2,7-NAF.P-OH) (–CH2), δ = 4.04–4.06 and δ = 4.26–4.29; (–CH3), δ = 1.34 ppm; (–OH), δ = 2.51 ppm; (Ar), δ = 7.02–7.05 and δ = 7.66–7.68 ppm.
(2,7-NAF.P-EP) (–CH2), δ = 4.04–4.05 and δ = 4.27–4.28; (–CH3), δ = 1.33–1.34 ppm; (–OH), δ = 2.45 ppm; (epoxy), δ = 2.65–5.66 and δ = 2.81–2.82 ppm, (–O–CH2–) = 3.19, 3.91 ppm. (Ar), δ = 7.04–7.05 and δ = 7.65–7.67 ppm.
(2,7-NAF.PMEM) (–CH2) δ = 4.04–4.06 ppm, δ = 4.25–4.27; (–CH3) δ = 2.17 ppm; (–OH), δ = 2.66 ppm; (–CH = CH2), δ = 5.57–5.58; δ = 6.13–6.15 ppm; (Ar) δ = 7.03–7.05 and δ = 7.65–7.68 ppm.
13 C-NMR
(2,7-NAF.P-OH) (CH3)– 18.76 ppm; –CH– 66.27 ppm; –CH2– 73.27 ppm; naphthalene ring 106.37, 116.23, 124.6, 129.25, 135.72, and 157.13 ppm.
(2,7-NAF.P-EP) (CH3)– 18.8 ppm; –CH–, 66.25 ppm; –CH2–, 71.68, 73.27 ppm; epoxide group 44.39–44.50, 51.04–51.09 ppm naphthalene ring, 106.36, 116.23, 124.62, 129.24, 135.74, and 157.08–157.36 ppm.
(2,7-NAF.PMEM) (2,7-NAF.P-EP): (CH3)– 18.27, 18.80 ppm; –CH–, 66.25 ppm; –CH2–, 71.68, 73.26 ppm; C=O 167.39 ppm; CH2 = CH(CH3)– 26.0, 135.97 ppm, naphthalene ring 106.36, 116.23, 124.61, 129.23, 135.71, and 157.14 ppm.
Thermal properties
The data obtained from the DSC, TG, and DTG measurements are shown in Table 1 and Figs. 5, 6 and 7. Data in Table 1 indicate that the thermal properties of the copolymers containing MMA and those containing St are significantly different. The copolymer containing St (1:20) has 5 % mass loss temperature about 80 °C higher than MMA copolymer.
The DSC curves of cross-linked copolymers are presented in Fig. 5, which also shows some differences in the thermal behavior of the copolymers studied. In the curves, only one endothermic effect connected with thermal degradation is visible at 375 and 376 °C for MMA copolymers and at 411 and 416 °C for St copolymers, detected in T peak. The position of those peaks confirms the results obtained in the TG/DTG analysis. The appearance of those peaks is a result of the decomposition of copolymers under pyrolysis. The influence of the amount of monomer (1:5 and 1:20) is rather small. Larger differences are observed when one compares the chemical nature of the monomers used (St and MMA). Decomposition of MMA copolymers occurs at temperatures about 40 °C lower than those for St derivatives. This is connected with the chemical structure of the monomers and especially the presence of aromatic rings in the 2,7-NAF.PMEM-St system.
Figures 6 and 7 show TG/DTG curves. It can be seen that the starting mass loss of the samples ranges from 200 to 220 °C. The copolymers have good thermal resistance especially in comparison with other methacrylate derivatives [19–21]. In total, 5 % mass loss for MMA is observed at T 5% = 250 °C, while St copolymers show T 5% at much higher temperature of 352 °C (2,7-NAF.PMEM-St, 1:20). The final decomposition temperature (T f) is in the range of 450–600 °C. The thermal stability of the copolymers becomes higher with an increase in St concentration. The DTG curves contain one separate degradation step. The maximum decomposition peak is observed in the range of 280–440 °C, with a maximum mass loss at T max = 377–418 °C and is related to the total degradation of aromatic fragments of copolymers. At T max, the samples were practically completely decomposed.
The QMS spectra collected at 20–600 °C during pyrolysis of the copolymers are presented in Fig. 8. Some differences between MS spectra for MMA and St copolymers are visible. The presence of m/z ions observed in both spectra (18, 44) indicates the formation of water vapor and carbon dioxide. The presence of 28 m/z ions in the MS profile is associated with the formation of carbon mono oxide, which confirms the presence of oxygen functional groups in the structure of the copolymers. The QMS analysis of MMA copolymers points to the formation of ions and their fragments characteristic of alkene (14, 32, 39, 67) and methacrylic groups (42). During the decomposition, the aromatic derivative (naphthalene) evolves fragmentary ions (m/z 52, 56, 59, 78, 81, 84, 99).
The QMS spectra for St copolymers point to the presence of typical decomposition products of aromatic esters, which are also observed during pyrolysis. The presence of m/z ions characteristic of aromatic fragments (52, 63, 89, 91), phenol derivatives (78), and St (104) is visible. The presence of m/z ions characteristic of aliphatic group is also evident (14, 38).
Photoluminescent properties
The UV–Vis absorption spectra of 2,7-NAF.PMEM-MMA and 2,7-NAF.PMEM-St are given in Fig. 9. The absorption bands at 330 nm originate from naphthalene [10], whereas the 450-nm band is characteristic of π conjugated derivatives of naphthalene. Polymer concentration has no influence on the position of the absorption bands.
The excitation and luminescence spectra of 2,7-NAF.PMEM-MMA and 2,7-NAF.PMEM-St are given in Figs. 10 and 11, respectively. The luminescence band at 510 nm, which is more intense for the St system, is in the region characteristic of luminescence materials based on naphthalene [22, 23]. It probably results from the π conjugation in the naphthalene-diepoxymethacrylate moiety. The lack of a band shift with polymer concentration probably results from the absence of any strong interactions among the chains of the polymer and the lack of a π conjugation along the polymer backbone.
A comparison of the luminescence properties of the previously synthesized naphthalene copolymers [16] and the present material leads to the conclusion that an introduction of propylene groups to the aliphatic chain results in the weakening of photoluminescent properties. Interestingly, luminescent bands are red shifted by about 15 nm when the new polymer is compared with 2,7-NAF.DM. This is probably caused by quenching of excitation energy as a result of thermal oscillation of the longer substituents in naphthalene.
Conclusions
The new monomer of a methacrylic derivative of naphthalene-2,7-diol was obtained as a result of a three-step reaction with propylene carbonate, 2-(chloromethyl)oxirane, and methacrylic acid. In the next step, thermal copolymerization of the new monomer with St and MMA was carried out.
The chemical structure of the obtained copolymers affected their thermal properties. In the DSC curves, one exothermic effect was visible at 375 and 376 °C for MMA (1:5, 1:20) copolymers and at 411 and 416 °C for St (1:5, 1:20) copolymers, which was connected with the total degradation of the copolymers.
TG/DTG data revealed that the copolymers had good thermal properties: 5 % mass loss was observed at 250 °C for MMA and at 352 °C for St copolymers. The final decomposition temperature could be as high as 600 °C. With the increase in St concentration, the thermal resistance of the copolymers also increased, paralleled by an increase in photoluminescence intensity.
References
Mori T, Kijima M. Synthesis and electroluminescence properties of carbazole-containing 2,6-naphthalene-based conjugated polymers. Eur Polym J. 2009;45:1149–57.
Feng J, Chen X, Han Q, Wang H, Lu P, Wang Y. Naphthalene-based fluorophores: synthesis characterization, and photophysical properties. J Lumin. 2011;131:2775–83.
Sannasi V, Sundararaj BG, Meenakshi S, Jeyakumar D. Synthesis, characterization and optical properties of poly(4,4′-dioctyloxy-3,3′-biphenylene vinylene) copolymers. Iran Polym J. 2011;20:633–44.
Ahn T. Systematic approaches for blue light-emitting polymers by introducing various naphthalene linkages into carbazole containing PPV derivatives. Trans Electr Electron Mater. 2013;14:258–62.
Zerza G, Röthler B, Sariciftci NS, Gómez R, Segura JL, Martín N. Photophysical properties and optoelectronic device applications of a novel naphthalene–vinylene type conjugated polymer. J Phys Chem B. 2001;105:4099–104.
Feng L, Chen Z. Light-emitting conjugated molecule containing 1,3,4-oxadiazole, carbazole and naphthalene units. Spectrochim Acta A. 2006;63:15–20.
Zhao X, Wang C, Cheng Y, Chen W, Zhu M. Novel photoluminescence poly(fluorinated imide)selectrospun fibers with blue, olive green and red fluorescence. Colloid Polym Sci. 2010;288:1471–7.
Nehls BS. Naphthalene based conjugated materials. Dissertation zur Erlangung des akademischen Grades Doktor der Naturwissenschaften (Dr. rer. nat., eingereicht im Fachbereich C – Mathematik und Naturwissenschaften der Bergischen Universität Wuppertal, Wuppertal, 2005).
Hohloch M, Segura JL, Dötinger SE, Hohnholz D, Steinhuber E, Spreitzer H, Hanack M. Design, synthesis and study of photoluminescence and electroluminescence of new poly(2,6- naphthylenevinylene) derivatives. Synth Met. 1997;84:319–22.
Yamamoto T, Lee BL. New soluble, coplanar poly(naphthalene-2,6-diyl)-type π-conjugated polymer, poly(pyrimido[5,4-d]pyrimidine-2,6-diyl), with nitrogen atoms at all of the o-positions. Synthesis, solid structure, optical properties, self-assembling phenomena, and redox behavior. Macromolecules. 2002;35:2993–9.
Tiwari B, Sharma S. Synthesis and thermal stability of conjugated polymer of 1,2-dichlorobenzene and naphthalene. Int J Metall Mater Sci Eng (IJMMSE). 2013;3:29–36.
Pschirer NG, Vaughn ME, Dong YB, Loye HC, Bunz UHF. Novel liquid-crystalline PPE-naphthalene copolymers displaying blue solid-state fluorescence. Chem Commun. 2000;2000:85–86.
Lynch P, O’Neill L, Bradley D, Byrne H, McNamara M. A systematic study of the effects of naphthalene and anthracene substitution on the properties of PPV derivative conjugated systems. Macromolecules. 2007;40:7895–901.
Mori T, Kijima M. Synthesis and optical properties of blue luminescent poly(2,6-naphthalene)s. J Polym Sci Part A Polym Chem. 2008;46:4258–63.
Podkościelna B. New photoluminescent copolymers of naphthalene-2,7-diol dimethacrylate and N-vinyl-2-pyrrolidone: synthesis, characterisation and properties. J Therm Anal Calorim. 2014;116:785–93.
Podkościelna B, Lipke A, Gawdzik B, Majdan M. Synthesis, characterization and luminescent properties of new copolymers of dimethacrylate derivatives of naphthalene-2,7-diol. Polym Adv Technol. 2015;26:176–81.
Podkościelna B, Gawdzik B. Influence of diluent compositions on the porous structure of methacrylate derivatives of aromatic diols and divinylbenzene. Appl Surf Sci. 2010;256:2462–7.
Podkościelna B. Method of photoluminescence copolymers preparation. Patent PL. (2015); P.402734.
Czech Z, Kowalczyk A, Ragańska P, Antosik A. Thermal stability and degradation of selected poly(alkyl methacrylates) used in the polymer industry. J Therm Anal Calorim. 2015;119:1157–61.
Hussein MA, El-Shishtawy RM, Abu-Zied BM, Asiri AM. The impact of cross-linking degree on the thermal and texture behavior of poly(methyl methacrylate). J Therm Anal Calorim. 2016;. doi:10.1007/s10973-016-5240-1.
Podkościelna B. Synthesis, spectroscopic and thermal characterization of the new photoluminescent monomer 2,7-di(methacryloyloxy)naphthalene and its copolymerization with selected vinyl monomers. J Therm Anal Calorim. 2016;123:273–82.
Sannasi V, Manikandan P, Sundararaj BG, Vijayan MT, Jeyakumar D. Synthesis of alternate-block copolymers of poly(2,5-dioctyloxy phenylene vinylene)s with varying positional naphthalene moieties. Iran Polym J. 2010;19:969–81.
Behnisch B, Martinez-Ruiz P, Schweikart KH, Hanack M. Luminescence of new 2,6- and 1,5-naphthalene-based PPV-type trimers and polymers. Eur J Org Chem. 2000;2000:2541–9.
Acknowledgements
The research was carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Operational Program Development of Eastern Poland 2007–2013 (Contract No. POPW.01.03.00-06-009/11-00), Equipping the laboratories of the Faculties of Biology and Biotechnology, Mathematics, Physics and Informatics, and Chemistry for studies of biologically active substances and environmental samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Podkościelna, B., Lipke, A., Majdan, M. et al. Thermal and photoluminescence analysis of a methacrylic diester derivative of naphthalene-2,7-diol. J Therm Anal Calorim 126, 161–170 (2016). https://doi.org/10.1007/s10973-016-5587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5587-3