Abstract
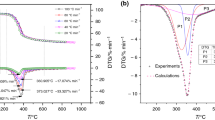
Pyrolysis behavior and kinetic properties of sawdust with the heating rates (β) of 5, 10, 15 and 20 °C min−1 in nitrogen atmosphere were analyzed by using a thermogravimetric analyzer. The results showed that organics of sawdust mainly decomposed at 250–400 °C, and greater heating rate can move the peak of differential thermogravimetric curves toward higher temperature. Two iso-conversional methods, Flynn–Wall–Ozawa (FWO) method and Kissinger–Akahira–Sunose (KAS) method, were employed to analyze the non-isothermal pyrolysis kinetics of sawdust, and the range of apparent activation energies for sawdust pyrolysis is between 101.53 and 114.83 kJ mol−1 using FWO method and is between 95.94 and 114.87 kJ mol−1 using KAS method. Error limit from heating rate was first proposed to examine the reliability of two iso-conversional methods, and the error from heating rate cannot exceed the range of ±20 kJ mol−1 in this study. Overall, these results suggested that the experimental results and kinetic parameters provided useful information for the design of pyrolytic processing system using sawdust as feedstock, and error limits demonstrated the precision of these obtained apparent activation energies.




Similar content being viewed by others
References
Jeguirim M, Trouve G. Pyrolysis characteristics and kinetics of Arundo donax using thermogravimetric analysis. Bioresour Technol. 2009;100:4026–31.
Kim SS, et al. Pyrolysis kinetics and decomposition characteristics of pine trees. Bioresour Technol. 2010;101:9797–802.
Zhao H, et al. Thermogravimetry study of pyrolytic characteristics and kinetics of the giant wetland plant Phragmites australis. J Therm Anal Calorim. 2012;110:611–7.
Biney PO, et al. Kinetics of the pyrolysis of arundo, sawdust, corn stover and switch grass biomass by thermogravimetric analysis using a multi-stage model. Bioresour Technol. 2015;179:113–22.
Zhu FL, et al. Kinetics of pyrolysis of ramie fabric wastes from thermogravimetric data. J Therm Anal Calorim. 2015;119:651–7.
Guan YJ, et al. Co-pyrolysis behaviors of energy grass and lignite. Energy Convers Manag. 2015;93:132–40.
Qiu HW, Zhou QC, Geng J. Pyrolytic and kinetic characteristics of Platycodon grandiflorum peel and its cellulose extract. Carbohydr Polym. 2015;117:644–9.
Oyedun AO, et al. Thermogravimetric analysis of the pyrolysis characteristics and kinetics of plastics and biomass blends. Fuel Process Technol. 2014;128:471–81.
Mu L, et al. Pyrolysis behaviors and kinetics of refining and chemicals wastewater, lignite and their blends through TGA. Bioresour Technol. 2015;180:22–31.
Naya Sebio-Puñal S, et al. Thermogravimetric analysis of wood, holocellulose, and lignin from five wood species. J Therm Anal Calorim. 2012;109:1163–7.
Naskar M, Chaki TK, Reddy KS. Effect of waste plastic as modifier on thermal stability and degradation kinetics of bitumen/waste plastics blend. Thermochim Acta. 2010;509:128–34.
Park SS, et al. Study on pyrolysis characteristics of refuse plastic fuel using lab-scale tube furnace and thermogravimetric analysis reactor. J Anal Appl Pyrolysis. 2012;97:29–38.
Sharara MA, et al. Pyrolysis kinetics of algal consortia grown using swine manure wastewater. Bioresour Technol. 2014;169:658–66.
Cheng G, et al. Pyrolysis of ramie residue: kinetic study and fuel gas produced in a cyclone furnace. Bioresour Technol. 2011;102:3451–6.
Poletto M, Zattera AJ, Santana RMC. Thermal decomposition of wood: kinetics and degradation mechanisms. Bioresour Technol. 2012;126:7–12.
Tapasvi D, et al. Thermal Decomposition Kinetics of Woods with an Emphasis on Torrefaction. Energy Fuels. 2013;27:6134–45.
Ren S, Zhang JL. Thermogravimetric analysis of anthracite and waste plastics by iso-conversional method. Thermochim Acta. 2013;561:36–40.
Saha B, Maiti AK, Ghoshal AK. Model-free method for isothermal and non-isothermal decomposition kinetics analysis of PET sample. Thermochim Acta. 2006;444:46–52.
Deng N, Zhang YF, Wang Y. Thermogravimetric analysis and kinetic study on pyrolysis of representative medical waste composition. Waste Manag. 2008;28:1572–80.
Papageorgiou DG, Bikiaris DN, Chrissafis K. Effect of crystalline structure of polypropylene random copolymers on mechanical properties and thermal degradation kinetics. Thermochim Acta. 2012;543:288–94.
Yan JH, et al. Analysis of volatile species kinetics during typical medical waste materials pyrolysis using a distributed activation energy model. J Hazard Mater. 2009;162:646–51.
Yu H, et al. Thermogravimetric analysis and kinetic study of bamboo waste treated by Echinodontium taxodii using a modified three-parallel-reactions model. Bioresour Technol. 2015;185:324–30.
Aboulkas A, et al. Investigation on pyrolysis of Moroccan oil shale/plastic mixtures by thermogravimetric analysis. Fuel Process Technol. 2008;89:1000–6.
Wang JX, Zhao HB. Pyrolysis kinetics of perfusion tubes under non-isothermal and isothermal conditions. Energy Convers Manag. 2015;106:1048–56.
Chan O, Valix M. Thermogravimetric analysis and kinetic study of biochemically pretreated printed circuit board wastes. Waste Biomass Valoriz. 2014;5:211–22.
Wang G, et al. TG study on pyrolysis of biomass and its three components under syngas. Fuel. 2008;87:552–8.
Singh S, Wu CF, Williams PT. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. J Anal Appl Pyrolysis. 2012;94:99–107.
Kim SS, et al. Non-isothermal pyrolysis of the mixtures of waste automobile lubricating oil and polystyrene in a stirred batch reactor. Renew Energy. 2013;54:241–7.
Seidelt S, Muller-Hagedorn A, Bockhorn H. Description of tire pyrolysis by thermal degradation behaviour of main components. J Anal Appl Pyrolysis. 2006;75:11–8.
Massaro MM, Son SF, Groven LJ. Mechanical, pyrolysis, and combustion characterization of briquetted coal fines with municipal solid waste plastic (MSW) binders. Fuel. 2014;115:62–9.
Vyazovkin S, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Janković B. Kinetic analysis of the nonisothermal decomposition of potassium metabisulfite using the model-fitting and isoconversional (model-free) methods. Chem Eng J. 2008;139:128–35.
Bai F, et al. Kinetic study on the pyrolysis behavior of Huadian oil shale via non-isothermal thermogravimetric data. Fuel. 2015;146:111–8.
Acknowledgements
These authors were supported by the Youth Top–notch Talent Support Program of the Central Organization Department (51522603).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Zhao, H. Error evaluation on pyrolysis kinetics of sawdust using iso-conversional methods. J Therm Anal Calorim 124, 1635–1640 (2016). https://doi.org/10.1007/s10973-016-5308-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5308-y