Abstract
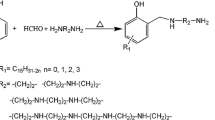
The purpose of this study is to investigate the curing kinetics of neat and pre-crosslinked or non-crosslinked carboxyl-terminated butadiene acrylonitrile liquid rubber (CTBN) toughened epoxies cured with isophorone diamine (IPDA) using non-isothermal and isothermal differential scanning calorimetry. The results show that CTBN has a complex effect on the curing process of epoxy/IPDA systems. The carboxyl groups of CTBN can accelerate the curing reactions, while the phase-separated CTBN particles and the dilution of curing ingredients caused by CTBN addition can hinder the curing reactions, resulting in an increase in activation energy. Interestingly, the activation energy of pre-crosslinked CTBN/epoxy systems decreases compared to their non-crosslinked counterparts because of the enhanced curing effect of the benzoic acid produced by benzoyl peroxide (BPO). An autocatalytic Kamal’s model is successfully used to describe the curing process of epoxy systems studied.







Similar content being viewed by others
References
May CA, Tanaka Y. Epoxy resins: chemistry and technology. 1st ed. New York: Marcel Dekker Inc.; 1973.
Roşu D, Mustaţă F, Caşcaval CN. Investigation of the curing reactions of some multifunctional epoxy resins using differential scanning calorimetry. Thermochim Acta. 2001;370(1–2):105–10. doi:10.1016/s0040-6031(00)00787-5.
Galy J, Sabra A, Pascault J-P. Characterization of epoxy thermosetting systems by differential scanning calorimetry. Polym Eng Sci. 1986;26(21):1514–23.
Garg AC, Mai Y-W. Failure mechanisms in toughened epoxy resins—A review. Compos Sci Technol. 1988;31(3):179–223. doi:10.1016/0266-3538(88)90009-7.
Chen W, Li P, Yu Y, Yang X. Curing kinetics study of an epoxy resin system for T800 carbon fiber filament wound composites by dynamic and isothermal DSC. J Appl Polym Sci. 2008;107(3):1493–9. doi:10.1002/app.26861.
Du S, Guo Z-S, Zhang B, Wu Z. Cure kinetics of epoxy resin used for advanced composites. Polym Int. 2004;53(9):1343–7. doi:10.1002/pi.1533.
Karayannidou EG, Achilias DS, Sideridou ID. Cure kinetics of epoxy–amine resins used in the restoration of works of art from glass or ceramic. Eur Polym J. 2006;42(12):3311–23. doi:10.1016/j.eurpolymj.2006.08.025.
Romo-Uribe A, Arcos-Casarrubias JA, Flores A, Valerio-Cárdenas C, González AE. Influence of rubber on the curing kinetics of DGEBA epoxy and the effect on the morphology and hardness of the composites. Polym Bull. 2014;71(5):1241–62. doi:10.1007/s00289-014-1121-6.
Yoo MJ, Kim SH, Park SD, Lee WS, Sun J-W, Choi J-H, et al. Investigation of curing kinetics of various cycloaliphatic epoxy resins using dynamic thermal analysis. Eur Polym J. 2010;46(5):1158–62. doi:10.1016/j.eurpolymj.2010.02.001.
Zukas WX. Monitoring the cure of an epoxy-anhydride resin. Polym Eng Sci. 1989;29(22):1553–9.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520(1–2):1–19. doi:10.1016/j.tca.2011.03.034.
Cai H, Li P, Sui G, Yu Y, Li G, Yang X, et al. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim Acta. 2008;473(1–2):101–5. doi:10.1016/j.tca.2008.04.012.
Malek J. The kinetic analysis of non-isothermal data. Thermochim Acta. 1992;200:257–69.
Saad GR, Abd Elhamid EE, Elmenyawy SA. Dynamic cure kinetics and thermal degradation of brominated epoxy resin–organoclay based nanocomposites. Thermochim Acta. 2011;524(1–2):186–93. doi:10.1016/j.tca.2011.07.014.
Boey FYC, Qiang W. Experimental modeling of the cure kinetics of an epoxy-hexaanhydro-4-methylphthalicanhydride (MHHPA) system. Polymer. 2000;41:2081–94.
Zhao K, Wang J, Song X, Liang C, Xu S. Curing kinetics of nanostructured epoxy blends toughened with epoxidized carboxyl-terminated liquid rubber. Thermochim Acta. 2015;605:8–15. doi:10.1016/j.tca.2015.02.007.
Zhang C, Liu X, Cheng J, Zhang J. Study on curing kinetics of diglycidyl 1,2-cyclohexane dicarboxylate epoxy/episulfide resin system with hexahydro-4-methylphthalic anhydride as a curing agent. J Therm Anal Calorim. 2015;120(3):1893–903. doi:10.1007/s10973-015-4527-y.
Li C, Liu M-H, Liu Z-Y, Qing M-L, Wang G. DSC and curing kinetics of epoxy resin using cyclohexanediol diglycidyl ether as active diluents. J Therm Anal Calorim. 2013;116(1):411–6. doi:10.1007/s10973-013-3471-y.
Tripathi G, Srivastava D. Cure kinetics of ternary blends of epoxy resins studied by nonisothermal DSC data. J Appl Polym Sci. 2009;112(5):3119–26. doi:10.1002/app.29781.
Thomas R, Sinturel C, Pionteck J, Puliyalil H, Thomas S. In-situ cure and cure kinetic analysis of a liquid rubber modified epoxy resin. Ind Eng Chem Res. 2012:120913092228002. doi:10.1021/ie2029927.
Vyazovkin S. Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules. 1996;29:1867–73.
Pezzati E, Baldini P, Schiraldi A. Epoxy polymers: effect of the elastomer in the kinetics of polymerization. Thermochim Acta. 1987;122:29–35.
Szeluga U, Kurzeja L, Galina H. Curing of epoxy/novolac system modified with reactive liquid rubber and carbon filler. Polym Bull. 2008;60(4):555–67. doi:10.1007/s00289-008-0889-7.
Calabrese L, Valenza A. Effect of CTBN rubber inclusions on the curing kinetic of DGEBA–DGEBF epoxy resin. Eur Polym J. 2003;39(7):1355–63. doi:10.1016/s0014-3057(02)00390-7.
Wise CW, Cook WD, Goodwin AA. CTBN rubber phase precipitation in model epoxy resins. Polymer. 2000;41(12):4625–33. doi:10.1016/s0032-3861(99)00686-2.
Thomas R, Durix S, Sinturel C, Omonov T, Goossens S, Groeninckx G, et al. Cure kinetics, morphology and miscibility of modified DGEBA-based epoxy resin—effects of a liquid rubber inclusion. Polymer. 2007;48(6):1695–710. doi:10.1016/j.polymer.2007.01.018.
Vijayan PP, Puglia D, Jyotishkumar P, Kenny JM, Thomas S. Effect of nanoclay and carboxyl-terminated (butadiene-co-acrylonitrile) (CTBN) rubber on the reaction induced phase separation and cure kinetics of an epoxy/cyclic anhydride system. J Mater Sci. 2012;47(13):5241–53. doi:10.1007/s10853-012-6409-z.
Hsu Y-G, Liang C-W. Properties and behavior of CTBN-modified epoxy with IPN structure. J Appl Polym Sci. 2007;106(3):1576–84. doi:10.1002/app.25412.
Zhou H-S, Song X-X, Xu S-A. Mechanical and thermal properties of novel rubber-toughened epoxy blend prepared by in situ pre-crosslinking. J Appl Polym Sci. 2014;. doi:10.1002/app.41110.
Zhou H, Xu S. A new method to prepare rubber toughened epoxy with high modulus and high impact strength. Mater Lett. 2014;121:238–40. doi:10.1016/j.matlet.2014.01.160.
Tripathi M, Kumar D, Rajagopal C, Roy PK. Curing kinetics of self-healing epoxy thermosets. J Therm Anal Calorim. 2014;119(1):547–55. doi:10.1007/s10973-014-4128-1.
Hong S-G, Chan C-K. The curing behaviors of the epoxy/dicyanamide system modified with epoxidized natural rubber. Thermochim Acta. 2004;417(1):99–106. doi:10.1016/j.tca.2003.12.015.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6. doi:10.1021/ac60131a045.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2(3):301–24. doi:10.1007/bf01911411.
Bai Y, Yang P, Zhang S, Li Y, Gu Y. Curing kinetics of phenolphthalein–aniline-based benzoxazine investigated by non-isothermal differential scanning calorimetry. J Therm Anal Calorim. 2015;120(3):1755–64. doi:10.1007/s10973-015-4544-x.
Ton-That MT, Ngo TD, Ding P, Fang G, Cole KC, Hoa SV. Epoxy nanocomposites: analysis and kinetics of cure. Polym Eng Sci. 2004;44(6):1132–41. doi:10.1002/pen.20106.
Martinez I, Martin MD, Eceiza A, Oyanguren P, Mondragon I. Phase separation in polysulfone-modified epoxy mixtures. Relationships between curing conditions, morphology and ultimate behavior. Polymer. 2000;41(3):1027–35. doi:10.1016/s0032-3861(99)00238-4.
Jenninger W. Calorimetric studies of isothermal curing of phase separating epoxy networks. Polymer. 2000;41(4):1577–88. doi:10.1016/s0032-3861(99)00274-8.
Zaman F, Beezer AE, Mitchell JC, Clarkson Q, Elliot J, Davis AF, et al. The stability of benzoyl peroxide by isothermal microcalorimetry. Int J Pharm. 2001;227(1–2):133–7. doi:10.1016/s0378-5173(01)00791-8.
Liu S-H, Hou H-Y, Shu C-M. Thermal hazard evaluation of the autocatalytic reaction of benzoyl peroxide using DSC and TAM III. Thermochim Acta. 2015;605:68–76. doi:10.1016/j.tca.2015.02.008.
Hu Y, Wu L. Curing agent. Beijing: Chemical Industry Press; 2004.
Prime RB. Differential scanning calorimetry of the epoxy cure reaction. Polym Eng Sci. 1973;13(5):365–71. doi:10.1002/pen.760130508.
Acitelli MA, Prime RB, Sacher E. Kinetics of epoxy cure: (1) the system bisphenol-A diglycidyl ether/m-phenylene diamine. Polymer. 1971;12(5):335–43. doi:10.1016/0032-3861(71)90056-5.
Kamal MR, Sourour S. Kinetics and thermal characterization of thermoset cure. Polym Eng Sci. 1973;13(1):59–64.
Sourour S, Kamal MR. Differential scanning calorimetry of epoxy cure: isothermal cure kinetics. Thermochim Acta. 1976;14(1–2):41–59. doi:10.1016/0040-6031(76)80056-1.
De Nograro FF, Guerrero P, Corcuera MA, Mondragon I. Effects of chemical structure of hardener on curing evolution and on the dynamic mechanical behavior of epoxy resins. J Appl Polym Sci. 1995;56(2):177–92. doi:10.1002/app.1995.070560208.
Acknowledgements
This research was supported by the National Science Foundation of China (No. 51073052) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, X., Xu, S. Curing kinetics of pre-crosslinked carboxyl-terminated butadiene acrylonitrile (CTBN) modified epoxy blends. J Therm Anal Calorim 123, 319–327 (2016). https://doi.org/10.1007/s10973-015-4989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4989-y