Abstract
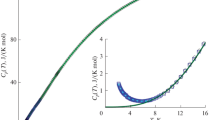
Heat capacity measurements have been conducted by means of DSC on both crystalline and glassy lithium metaphosphate, from room temperature up to the melting region. The heat capacity of the glass is slightly higher than that of the crystal. Contrary to the crystal, in the neighborhood of T g, C p increases rapidly by 10 J mol−1 K−1 conferring to this glass a “fragile character.” Nevertheless, the passage through T m does not show any discontinuity and the values of the glass and of the crystal are identical. The Debye model appears to be realistic to describe the glass heat capacity to temperature dependence. The Debye temperature and frequency were determined by minimizing the R p and χ 2 parameters of the C v fitting curve. From the calculation of the entropy of the liquid at T > T m, the excess entropy of the glass at T g was determined. Using the dependence of the glass transition on the heating rate, we calculated the values of the activation energy for structural relaxation (E relax) and of the lower limit of the glass transition temperature (\( {T_{\text{g}}^{{^\circ }} } \)) which is a thermodynamic parameter, contrary to T g which is a kinetic parameter.





Similar content being viewed by others
References
Donald IW. Preparation, properties and chemistry of glass- and glass-ceramic-to-metal seals and coatings. J Mater Sci. 1993;28:2841–86.
Morena R. Phosphate glasses as alternatives to Pb-based sealing frits. J Non Cryst Solids. 2000;263–264:382–7.
Ehrt D, Seeber W. Glass for high performance optics and laser technology. J Non Cryst Solids. 1991;129:19–30.
Campbell JH, Suratwala TI. Nd-doped phosphate glasses for high-energy/high-peak-power lasers. J Non Cryst Solids. 2000;263–264:318–41.
Franks K, Abrahams I, Georgiou G, Knowles JC. Investigation of thermal parameters and crytallisation in a ternary CaO–Na2O–P2O5-based glass system. Biomaterials. 2001;22(5):497–501.
Abou Neel EA, Pickup DM, Valappil SP. Bioactive functional materials: a perspective on -based glasses. J Mater Chem. 2009;19:690–701.
Money BK, Hariharan K. Lithium ion conduction in lithium metaphosphate based systems. Appl Phys. 2007;A88:647–52.
Money BK, Hariharan K. Crystallization kinetics and phase transformation in superionic lithium metaphosphate (Li2O–P2O5) glass system. J Phys: Condens Matter. 2009;21:115102.
Inaba S, Oda S, Morinaga K. Heat capacity of oxide glasses measured by AC calorimetry. J Non Cryst Solids. 2002;306:42–9.
Inaba S, Oda S, Morinaga K. Heat capacity of oxide glasses at high temperature region. J Non Cryst Solids. 2003;325:258–66.
Lasocka M. The effect of scanning rate on glass transition temperature of splat-cooled Te85Ge15. J Mater Sci Eng. 1976;23:173–7.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1976;9:369–73.
Hill JO. For better thermal analysis and calorimetry. 3rd ed. ICTAC; 1991.
Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A. 1976;A32:751–67.
Rocherullé J, Trochet F, Marchand R. Kinetics of the NaPO3 glass devitrification studied by differential thermal analysis and X-ray powder diffraction. Key Eng Mater. 2002;206–213:2045–8.
Makishima A, Mackenzie JD. Direct calculation of Young’s modulus of glass. J Non-Cryst Solids. 1973;12:35–45.
Rocherullé J, Ecolivet C, Poulain M, Verdier P, Laurent Y. Elastic moduli of oxynitride glasses. Extension of Makishima and Mackenzie’s theory. J Non Cryst Solids. 1989;108:187–93.
Kingery WD, Bowen HK, Uhlmann DR. Introduction to ceramics. 2nd ed. Singapore: Wiley; 1991. p. 589.
Rocherullé J, Matecki M, Delugeard Y. Heat capacity measurements of MgYSiAlON glasses. J Non Cryst Solids. 1998;238:51–6.
Angell CA. Strong and fragile liquids. In: Ngai K, Wright GB, editors. Relaxation in complex systems. Springfield: US Dpt of Commerce; 1985.
Laughlin WT, Uhlmann DR. Viscous flow in simple organic liquids. J Phys Chem. 1972;76(16):2317–25.
Lewis GN, Randall M. Thermodynamics. 2nd ed. New York: Mc Graw-Hill; 1961.
Martin RA, Twyman HL, Rees GJ, Smith JM, Barney ER, Smith ME, Hanna JV, Newport RJ. A structural investigation of the alkali metal site distribution within bioactive glass using neutron diffraction and multinuclear solid state NMR. Phys Chem Chem Phys. 2012;14:12105–13.
Hudgens JJ. The structure and properties of anhydrous, alkali ultra-phosphate glasses. Retrospective theses and dissertations, Paper 11267; 1994.
Wers E, Oudadesse H. Thermal behaviour and excess entropy of bioactive glasses and Zn-doped glasses. J Therm Anal Calorim. 2014;115(3):2137–2144
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocherullé, J., Massera, J., Oudadesse, H. et al. Heat capacities of crystalline and glassy lithium metaphosphate up to the transition region. J Therm Anal Calorim 123, 401–407 (2016). https://doi.org/10.1007/s10973-015-4938-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4938-9