Abstract
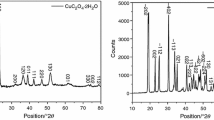
In this study, we present the synthesis of Cu–Cr–Pb nanocomposites by a simple co-precipitation method and evaluate the catalytic performance of the as-fabricated Cu–Cr–Pb nanocomposites on the thermal decomposition of ammonium perchlorate (AP). The X-ray diffraction, scanning electron microscopy, transmission electron microscopy and thermogravimetric analysis/differential scanning calorimetric techniques were employed to characterize the crystal phases, sizes and catalytic properties of as-obtained Cu–Cr–Pb nanocomposites, respectively. The results revealed that dropping Pb in CuCr2O4 could form Cu–Cr–Pb nanocomposites. The structure, size and catalytic property of the as-synthesized Cu–Cr–Pb nanocomposites are closely relevant to the Cu/Pb molar ratio in the starting reactants. Noticeably, when Cu–Cr–Pb nanocomposites were synthesized under the condition of the molar ratio of Cu/Pb = 4:1, the dispersion is better, the size distribution is narrower, and the catalytic performance for AP is more effective than those of the particles obtained by other Cu/Pb molar ratios, which may lead to potential applications of Cu–Cr–Pb nanocomposites in AP-based solid propellants in future.







Similar content being viewed by others
References
Yang Y, Liu HY, Li FS, Zhang XY, Liu JX. Nanometer transition metal oxide and rare earth oxide catalysis on AP thermal decomposition. J Propuls Technol. 2006;27:92–6.
Al-Harthi A, Willimas A. Effect of fuel binder and oxidizer particle diameter on the combustion of ammonium perchlorate based propellants. Fuel. 1998;77:1451–68.
Brill TB, Budenz BT. Flash pyrolysis of ammonium perchlorate-hydroxyl-terminated-polybutadiene mixtures including selected additives. Prog Astronaut Aeronaut. 2000;185:3–32.
Vargeese AA, Muralidharan K. Kinetics and mechanism of hydrothermally prepared copper oxide nanorod catalyzed decomposition of ammonium nitrate. Appl Catal A Gen. 2012;447:171–7.
Sathiskumar PS, Thomas CR, Madras G. Solution combustion synthesis of nanosized copper chromite and its use as a burn rate modifier in solid propellants. Ind Eng Chem Res. 2012;51:10108–16.
Ma ZY, Li FS, Bai HP. Effect of Fe2O3 in Fe2O3/AP composite particles on thermal decomposition of AP and on burning rate of the composite propellant. Propellants Explos Pyrotech. 2006;31:447–51.
Xu YY, Chen DR, Jiao ML, Xue KY. CuO microflowers composed of nanosheets: synthesis, characterization, and formation mechanism. Mater Res Bull. 2007;42:1723–31.
Habibi MH, Fakhri F. Sol–gel combustion synthesis and characterization of nanostructure copper chromite spinel. J Therm Anal Calorim. 2014;115:1329–33.
Xu XL, Chen WK, Li JQ. First-principles study of O2 adsorption and dissociation on the CuCr2O4 (100) surface. J Mol Struct Theochem. 2008;860:18–23.
Hosseini SG, Alavi MA, Ghavi A, Toloti SJH, Agend F. Modeling of burning rate equation of ammonium perchlorate particles over Cu–Cr–O nanocomposites application of hybrid DOE/ANN methodologies. J Therm Anal Calorim. 2015;119:99–109.
Li W, Cheng H. Cu–Cr–O nanocomposites: synthesis and characterization as catalysts for solid state propellants. Solid State Sci. 2007;9:750–5.
Li R, Liu XX, Huang YC, Wang XJ. Synthesis of Ni, Ni-P and Ni-B nanoparticles and their catalytic effect on the thermal decomposition of ammonium perchlorate. J Solid Rocket Technol. 2008;31:607–11.
Liu LL, Li FS, Tan LH, Ming L, Yi Y. Effects of nanometer Ni, Cu, Al and NiCu powders on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech. 2004;29:34–8.
Sammour MH. Influence of the chemical interaction of ballistic modifiers on the chemical stability of double-base propellants. Propellants Explos Pyrotech. 1998;23:266–71.
Hosseini SG, Abazari R, Gavi A. Pure CuCr2O4 nanoparticles: synthesis, characterization and their morphological and size effects on the catalytic thermal decomposition of ammonium perchlorate. Solid State Sci. 2014;37:72–9.
Yang Y, Cao XF, Liu LL, Liu HY, Li FS. Catalysis of nanometer transition metals on the thermal decomposition of ammonium perchlorate. Chin J Energy Mater. 2005;13:273–7.
Eslami A, Hosseini SG, Bazrgary M. Improvement of thermal decomposition properties of ammonium perchlorate particles using some polymer coating agents. J Therm Anal Calorim. 2013;113:721–30.
Boldyrev VV. Thermal decomposition of ammonium perchlorate. Thermochim Acta. 2006;443:1–36.
Jia ZG, Ren DP, Wang QZ, Zhu RS. A new precursor strategy to prepare ZnCo2O4 nanorods and their excellent catalytic activity for thermal decomposition of ammonium perchlorate. Appl Surf Sci. 2013;270:312–8.
Chaturvedi S, Dave PN. Nano-metal oxide: potential catalyst on thermal decomposition of ammonium perchlorate. J Exp Nanosci. 2012;7:205–31.
Patil PR, Krishnamurthy VN, Joshi SS. Effect of nano-copper oxide and copper chromite on the thermal decomposition of ammonium perchlorate. Propellants Explos Pyrotech. 2008;33:266–70.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Fan RH, Lu HL, Sun KN, Wang WX, Yi XB. Kinetics of thermite reaction in Al-Fe2O3 system. Thermochim Acta. 2006;440:129–31.
Pourmortazavi SM, Hajimirsadeghi SS, Kohsari I, Fathollahi M, Hosseini SG. Thermal decomposition of pyrotechnic mixtures containing either aluminum or magnesium powder as fuel. Fuel. 2008;87:244–51.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Project No. 51206081).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hao, G., Liu, J., Liu, H. et al. Cu–Cr–Pb nanocomposites. J Therm Anal Calorim 123, 263–272 (2016). https://doi.org/10.1007/s10973-015-4924-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4924-2