Abstract
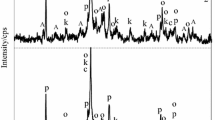
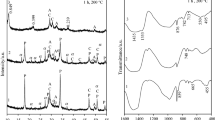
The influence of aluminum additive on the formation of α-C2SH and on kinetics of cementitious binder material at early stages of hydration was determined. α-C2SH was synthesized in the primary mixtures with CaO/(SiO2 + Al2O3) = 1.5 and Al2O3/(SiO2 + Al2O3) = 0; 0.025 and 0.05. The hydrothermal synthesis has been carried out in unstirred suspensions under saturated steam pressure in argon atmosphere at 175 °C temperature for 4, 8;,16, 24, 48 and 72 h by applying extra argon gas (10 bar). It was determined that in CaO–SiO2·nH2O–H2O suspensions within 4 h dicalcium silicate hydrates—α-C2S hydrate, C–S–H(II) and a low base semi-crystalline C–S–H(I) type calcium silicate hydrate—were formed. Meanwhile, Al2O3 additive changes the formation mechanism of synthesis products as well as their stability during the isothermal curing. It was observed that hydrogarnets formed after 4 h of hydrothermal treatment remained stable under all experimental conditions. It was determined that the addition of α-C2SH–Al in BM sample changed both the heat flow and the total quantity of heat released during early-stage hydration. It was determined that only 5 % of quartz reacts during the first 4.5 h of hydration in both BM samples, and the further reduction of its quantity depends on duration of process. The products of synthesis and hardening were characterized by simultaneous thermal analysis, microcalorimetry and X-ray diffraction analysis.









Similar content being viewed by others
References
Liu Z, Wang Z, Yuan MZ, Yu HB. Thermal efficiency modelling of the cement clinker manufacturing process. J Energy Inst. 2014. doi:10.1016/j.joei.2014.04.004.
Khurana S, Banerjee R, Gaitonde U. Energy balance and cogeneration for a cement plant. Appl Therm Eng. 2002;22:485–94.
Madlool NA, Saidur R, Hossain MS, Rahim NAA. Critical review on energy use and savings in the cement industries. Renew Sustain Energy Rev. 2011;15(4):2042–60.
Benhelal E, Zahedi G, Shamsaei G, Bahadori A. Global strategies and potentials to curb CO2 emissions in cement industry. J Clean Prod. 2013;51:142–61.
Swanepoel JA, Mathews EH, Vosloo J, Liebenberg L. Integrated energy optimisation for the cement industry: a case study perspective. Energ Convers Manage. 2014;78:765–75.
Peng J, Huang L, Zhao Y, Chen P, Zeng L, Zheng W. Modeling of carbon dioxide measurement on cement plants. Adv Mater Res. 2013;610–613:2120–2128.
Li C, Gong X, Cui S, Wang Z, Zheng Y, Chi B. CO2 emissions due to cement manufacture. Mater Sci Forum. 2011;685:181–7.
Turner KL, Collins FG. Carbon dioxide equivalent (CO2-e) emissions: a comparison between geopolymer and OPC cement concrete. Constr Build Mater. 2013;43:125–30.
Kacimi L, Martin C, Clastres P. Synthesis of α’ L-C2S cement from fly-ash using the hydrothermal method at low temperature and atmospheric pressure. J Hazard Mater. 2010;181(2):593–601.
Bell NS, Venigalla S, Gill PM, Adair JH. Morphological forms of tobermorite in hydrothermally treated calcium silicate hydrate gels. J Am Ceram Soc. 1996;79(8):2175–8.
Baltakys K, Siauciunas R. Formation of gyrolite in the CaO–quartz–Na2O–H2O system. Mater Sci Pol. 2007;25(4):1089–100.
Richardson IG. The calcium silicate hydrates. Cem Concr Res. 2008;38(2):137–58.
Zhang X, Chang W, Zhang T, Ong CK. Nanostructure of calcium silicate hydrate gels in cement paste. J Am Ceram Soc. 2000;83(10):2600–4.
Hara N, Chan CF, Mitsuda T. Formation of 14 Å tobermorite. Cem Concr Res. 1978;8(1):113–5.
Stemmermann P, Schweike U, Garbev K, Beuchle G. Celitement—a sustainable prospect for the cement industry. Cem Int. 2010;8:52–66.
Stemmermann P, Beuchle G, Garbev K, Schweike U. Celitement®—A new sustainable hydraulic binder based on calcium hydrosilicates. Proceedings of the 13th International Congress on the chemistry of cement. 2011; 158.
Baltakys K, Dambrauskas T, Siauciunas R, Eisinas A. α-C2SH synthesis in the mixtures with CaO/SiO2 = 1.5 and application as a precursor for binder material. Adv Cem Res. 2014; Submitted.
Taylor HFW, Bessey GE. Review of hydrothermal reactions in the system lime-silica-water. Mag Concr Res. 1950;2(4):15.
Heller L. The structure of dicalcium silicate α-hydrate. Acta Crystallogr. 1952;5(6):724.
Kalousek GL, Logiudice JS, Dodson VH. Studies on the lime-rich crystalline solid phases in the system lime-silica-water. J Am Ceram Soc. 1954;37(1):7.
Taylor HFW. The calcium silicate hydrates. In: Taylor HFW, editor. The chemistry of cements. London: Academic Press; 1964. p. 167.
Imlach BV, Taylor HFW. Prolonged hydrothermal treatment of cement mixes I. Curing in water under saturated steam pressure at 140–170°C. Brit Ceram Trans J. 1972;71(1):71.
Mitsuda T, Kobayakawa S, Toraya H. Characterization of hydrothermally formed CSH. The 8th International Congress on the Chemistry of Cement, Rio de Janeiro. 1986; 3:176.
Ishida H, Yamazaki S, Sasaki K, Okada Y, Mitsuda T. α-Dicalcium silicate hydrate—preparation, decomposed phase, and its hydration. J Am Ceram Soc. 1993;76(7):1707.
Garbev K, Gasharova B, Beuchle G, Kreisz S. First observation of α-Ca2[SiO3(OH)](OH)-Ca6[Si2O7][SiO4](OH)2 phase transformation upon thermal treatment in air. J Am Ceram Soc. 2008;91(1):263.
Baltakys K, Dambrauskas T, Siauciunas R, Eisinas A. Formation of α-C2S hydrate in the mixtures with CaO/SiO2 = 1.75 by hydrothermal treatment at 200 °C. Rom J Mater. 2014;44(1):109–15.
Baltakys K, Siauciunas R. Influence of gypsum additive on the gyrolite formation process. Cem Concr Res. 2010;40(3):376–83.
Baltakys K, Siauciunas R. The influence of γ-Al2O3 and Na2O on the formation of calcium silicate hydrates in the CaO–quartz–H2O system. Mater Sci Pol. 2007;25(1):185–98.
Bullard JW, Jennings HM, Livingston RA, Nonat A, Scherer GW, Schweitzer JS, Scrivener KL, Thomas JJ. Mechanisms of cement hydration. Cem Concr Res. 2011;41:1208–23.
Mostafa NY, Brown PW. Heat of hydration of high reactive pozzolans in blended cements: isothermal conduction calorimetry. Thermochim Acta. 2005;435:162–7.
Melchert MBM, Viana MM, Lemos MS, Dweck J, Buechler PM. Simultaneous solidification of two catalyst wastes and their effect on the early stages of cement hydration. J Therm Anal Calorim. 2011;105(2):625–33.
Chaipanich A, Nochaiya T. Thermal analysis and microstructure of Portland cement-fly ash-silica fume pastes. J Therm Anal Calorim. 2010;99(2):487–93.
Gruyaert E, Robeyst N, De Belie N. Study of the hydration of Portland cement blended with blast-furnace slag by calorimetry and thermogravimetry. J Therm Anal Calorim. 2010;102(3):941–51.
Dweck J, Ferreira da Silva PF, Buechler PM, Cartledge FK. Study by thermogravimetry of the evolution of ettringite phase during type II Portland cement. J Therm Anal Calorim. 2002;69(1):179–86.
Siauciunas R, Mikaliunaite J, Urbonas L, Baltakys K. Tribochemical and thermal activation of α-C2S hydrate as precursor for cementitious binders. J Therm Anal Calorim. 2014. doi:10.1007/s10973-014-3921-1.
De Weerdt K, Ben Haha M, Le Saout G, Kjellsen KO, Justnes H, Lothenbach B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem Concr Res. 2011;41(3):279–91.
Pacewska B, Wilinska I, Bukowska M. Hydration of cement slurry in the presence of spent cracking catalyst. J Therm Anal Calorim. 2000;60(1):71–8.
Shi C, Dau RL. Some factors affecting early hydration of alkalislag cements. Cem Concr Res. 1999;26:439–47.
Gruyaert E, Robeyst N, De Belie N. Study of the hydration of Portland cement blended with blast-furnace slag by calorimetry and thermogravimetry. J Therm Anal Calorim. 2010;102:941–51.
Evju C. Initial hydration of cementitious systems using a simple isothermal calorimeter and dynamic correction. J Therm Anal Calorim. 2003;71:829–40.
Acknowledgements
This research was funded by a Grant (No. MIP – 025/2014) from the Research Council of Lithuania.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baltakys, K., Eisinas, A. & Dambrauskas, T. The influence of aluminum additive on the α-C2S hydrate formation process. J Therm Anal Calorim 121, 75–84 (2015). https://doi.org/10.1007/s10973-015-4591-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4591-3