Abstract
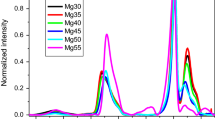
Raman spectra of xZnO (100−x)P2O5 (x = 35, 40, 45, 50, 55) glasses were described by the thermodynamic model of Shakhmatkin and Vedishcheva using the method of Malfait. The glasses were considered as the ideal solution of unreacted oxides and four compounds (ZnP2O6, Zn2P2O7, Zn3P2O8, and ZnP2O11). Due to missing thermodynamic data, thermodynamic model was based on the estimates of reaction Gibbs energies of formation of considered compounds obtained by fitting the 31P MAS NMR Q-distribution in ZnO–MoO3–P2O5 glasses. Simultaneously, the set of Raman spectra was analyzed by multivariate curve resolution method. The very good coincidence between the results of spectral decomposition based on the thermodynamic model on one side and on the results of MCR method on the other can be considered as plausible validation of the thermodynamic data used, i.e., as a general guideline for the situations where the thermodynamic data needed for the construction of Shakhmatkin and Vedishcheva thermodynamic model are missing but the detailed structural information is available.








Similar content being viewed by others
References
Šubčík J, Koudelka L, Mošner P, Montagne L, Tricot G, Delevoye L, Gregora I. Glass-forming ability and structure of ZnO–MoO3–P2O5 glasses. J Non-Cryst Solids. 2010;356:2509–16.
Shakhmatkin BA, Vedishcheva NM, Shultz MM, Wright AC. The thermodynamic properties of oxide glasses and glass-forming liquids and their chemical structure. J Non-Cryst Solids. 1994;177:249–56.
Vedishcheva NM, Shakhmatkin BA, Shultz MM, Wright AC. The thermodynamic modelling of glass properties: a practical proposition? J Non-Cryst Solids. 1996;196:239–43.
Shakhmatkin BA, Vedishcheva NM, Wright AC. Thermodynamics of borate melts and glasses. In: Wright AC, Feller SA, Hannon AC, editors. Borate glasses crystals and melts. Sheffield: Society of Glass. Technology; 1997. p. 189.
Shakhmatkin BA, Vedishcheva NM, Wright AC. Can thermodynamics relate the properties of melts and glasses to their structure? J Non-Cryst Solids. 2001;293–295:220–36.
Vedishcheva NM, Shakhmatkin BA, Wright AC. Thermodynamic modelling of the structure of glasses and melts: single-component, binary and ternary systems. J Non-Cryst Solids. 2001;293–295:312–7.
Vedishcheva NM, Shakhmatkin BA, Wright AC. Thermodynamic modelling of the structure of sodium borosilicate glasses. Phys Chem Glasses Eur J Glass Sci Technol B. 2003;44:191–6.
Vedishcheva NM, Shakhmatkin BA, Wright AC. The structure of sodium borosilicate glasses: thermodynamic modelling versus experiment. J Non-Cryst Solids. 2004;345&346:39–44.
Shakhmatkin BA, Vedishcheva NM, Wright AC. Thermodynamic modelling of the structure of oxyhalide glasses. J Non-Cryst Solids. 2004;345&346:461–8.
Liška M. Studying structure and thermal properties of oxide glasses. pp. 344–362, In: some thermodynamic, structural and behavioral aspects of materials accentuating non-crystalline states. University of West Bohemia in Pilsen, Nymburg 2009. ISBN 978-80-87269-06-0.
Liska M, Chromčíková M. Thermal properties and related structural and thermodynamic studies of oxide glasses. In: Šesták J, Holeček M, Málek J, editors. Glassy, amorphous and nano-crystalline materials: thermal physics, analysis, structure and properties. New York: Springer; 2011. p. 179–97.
High temperature glass melt property database for process modeling. Seward III T.P, Vascott T. editors. Amer Cer Society, Westerville, Ohio 2005.
Liška M, Macháček J, Chromčíková M, Gedeon O. Thermodynamic model and structure of ZnO-MoO3-P2O5 glasses. In: Book of Abstracts of the International Conference on Phosphate Glasses, Pardubice, 2014, p. 154 (www.icaris.cz/conf/borate-phosphate-2014).
Liška M, Macháček J, Chromčíková M, Gedeon O. Thermodynamic model and structure of ZnO-MoO3-P2O5 glasses. Eur. J. Glass Sci. Technology, Part B Phys. Chem. Glasses http://www.ingentaconnect.com/content/sgt/ejgst).
Schwarz J, Tichá H, Tichý L, Mertens R. Physical properties of PbO–ZnO–P2O5 glasses I. Infrared and Raman spectra. J Optoelectron Adv Mater. 2004;6:737–46.
Brow RK, Tallant DR, Myers ST, Phifer CC. The short-range structure of zinc polyphosphate glass. J Non-Cryst Solids. 1995;191:45–55.
Brow RK, Alam TM, Tallant DR, Kirkpatrick RJ. Spectroscopic studies on the structures of phosphate sealing glasses. MRS Bull. 1998;23:63–7.
Factor analysis Toolbox for MATLAB®. Applied Chemometrics, www.chemometrics.com (2014).
Malinowski ER. Factor analysis in chemistry. 3rd ed. New York: Wiley; 2002.
Zakaznova-Herzog VP, Malfait WJ, Herzog F, Halter WE. Quantitative Raman spectroscopy: principles and application to potassium silicate glasses. J Non-Cryst Solids. 2007;353:4015–28.
Malfait WJ, Zakaznova-Herzog VP, Halter WE. Quantitative Raman spectroscopy: high-temperature speciation of potassium silicate melts. J Non-Cryst Solids. 2007;353:4029–42.
Malfait WJ, Halter WE. Structural relaxation in silicate glasses and melts: high-temperature Raman spectroscopy. Phys Rev B. 2008;B77:014201.
Malfait WJ. Quantitative Raman spectroscopy: speciation of cesium silicate glasses. J Raman Spectrosc. 2009;40:1895–901.
Ruckebusch C, Blanchet L. Multivariate curve resolution: a review of advanced and tailored applications and challenges. Anal Chim Acta. 2013;765:28–36.
Meyer K. Characterization of the structure of binary zinc ultraphosphate glasses by infrared and Raman spectroscopy. J Non-Cryst Solids. 1997;209:227–39.
Acknowledgements
This work was supported by the Slovak Grant Agency for Science under the grant VEGA 1/0006/12 and by the Slovak Research and Development Agency Project ID: APVV-0487-11. This work was also supported by the Grant Agency of the Czech Republic through the Grant No. P108/10/1631.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liška, M., Lissová, M., Plško, A. et al. Thermodynamic model and Raman spectra of ZnO–P2O5 glasses. J Therm Anal Calorim 121, 85–91 (2015). https://doi.org/10.1007/s10973-015-4563-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4563-7