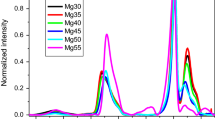
Abstract
The structure of glasses of the binary system CaO–P2O5 was investigated by the comparison of the results of the thermodynamic model of Shakhmatkin and Vedishcheva constructed using the molar Gibbs energies obtained from the FACT database with the results obtained by the analysis of Raman spectra of xCaO·(100 − x)P2O5 (x = 35, 40, 45, 50, 55) glasses performed by the principal component analysis (PCA) method and spectral decomposition by the method of Malfait. The PCA method identified three independent components in the studied spectral series. On the other hand, the thermodynamic modeling resulted in three components with significant abundance in the studied glasses, i.e., P2O5, CaP2O6, and Ca2P2O7. Using the method of Malfait, partial Raman spectra of P2O5, CaP2O6, and Ca2P2O7 were calculated. The experimental spectra were reproduced with very good accuracy. The multivariate curve resolution (MCR) method was used for the analysis of the experimental spectra. It was found that MCR loadings are practically identical with the partial Raman spectra obtained from the results of thermodynamic model by the method of Malfait. All the obtained results confirmed the structural information acquired from the thermodynamic model.






Similar content being viewed by others
References
Rao KJ. Structural chemistry of glasses. Amsterdam: Elsevier; 2002.
Varshneya AK. Fundamentals of inorganic glasses. Sheffield: Society of Glass Technology; 2006.
Brow RK. Review: the structure of simple phosphate glasses. J Non Cryst Solids. 2000;263&264:1–28.
Roiland C, Fayon F, Simon P, Massiot D. Characterization of the disordered phosphate network in CaO–P2O5 glasses by 31P solid state NMR and Raman spectroscopies. J Non Cryst Solids. 2011;357:1636–46.
Serena S, Carbajal L, Sainz AM, Caballero A. Thermodynamic assessment of the system CaO–P2O5: application of the ionic two-sublattice model to glass-forming melts. J Am Ceram Soc. 2011;94:3094–103.
Putlyaev VI, Safronova TV. A new generation of calcium phosphate biomaterials: the role of phase and chemical compositions. Glass Ceram. 2006;63:99–102.
Kokubo T, editor. Bioceramics and their clinical applications. Cambridge: Japan Medical Materials, Woodhead Publishing Ltd. and CRC Press; 2008.
Shakhmatkin BA, Vedishcheva NM, Shultz MM, Wright AC. The thermodynamic properties of oxide glasses and glass-forming liquids and their chemical structure. J Non-Cryst Solids. 1994;177:249–56.
Vedishcheva NM, Shakhmatkin BA, Shultz MM, Wright AC. The thermodynamic modelling of glass properties: a practical proposition? J Non-Cryst Solids. 1996;196:239–43.
Shakhmatkin BA, Vedishcheva NM, Wright AC. Can thermodynamics relate the properties of melts and glasses to their structure? J Non-Cryst Solids. 2001;293–295:220–36.
Vedishcheva NM, Shakhmatkin BA, Wright AC. Thermodynamic modelling of the structure of glasses and melts: single-component, binary and ternary systems. J Non-Cryst Solids. 2001;293–295:312–7.
Vedishcheva NM, Shakhmatkin BA, Wright AC. The structure of sodium borosilicate glasses: thermodynamic modelling vs. experiment. J Non-Cryst Solids. 2004;345&346:39–44.
Shakhmatkin BA, Vedishcheva NM, Wright AC. Thermodynamic modelling of the structure of oxyhalide glasses. J Non-Cryst Solids. 2004;345&346:461–8.
Liška M, Chromčíková M. Thermal properties and related structural and thermodynamic studies of oxide glasses. In: Šesták J, Holeček M, Málek J, editors. Glassy, amorphous and nano-crystalline materials: thermal physics, analysis, structure and properties, Chapter 11. New York: Springer; 2011. p. 179–97.
Chromčíková M, Liška M, Macháček J, Šulcová J. Thermodynamic model and structure of CaO–P2O5 glasses. J Therm Anal Calorim. 2013;144:757–89.
Vonka P, Leitner J. Calculation of chemical equilibria in heterogeneous multicomponent systems. Calphad. 1995;19:25–36.
Seward TP III, Vascott T, editors. High temperature glass melt property database for process modeling. Westerville, OH: American Ceramic Society; 2005.
http://www.crct.polymtl.ca/fact/. Sept 2014.
Zakaznova-Herzog VP, Malfait WJ, Herzog F, Halter WE. J Non-Cryst Solids. 2007;353:4015–28.
Malfait WJ, Zakaznova-Herzog VP, Halter WE. J Non-Cryst Solids. 2007;353:4029–42.
Malfait WJ, Halter WE. Phys. Rev. B. 2008;B77:014201.
Malfait WJ. Quantitative Raman spectroscopy: speciation of cesium silicate glasses. J Raman Spectrosc. 2009;40:1895–901.
Factor analysis Toolbox for MATLAB®. Applied Chemometrics, www.chemometrics.com September, 2014.
Malinowski ER. Factor analysis in chemistry. 3rd ed. New York: Wiley; 2002.
Kramer R. Chemometric techniques for quantitative analysis. New York: Marcel Dekker; 1998.
Ruckebusch C, Blanchet L. Multivariate curve resolution: a review of advanced and tailored applications and challenges. Anal Chim Acta. 2013;765:28–36.
http://www.eigenvector.com/courses/EigenU_MCR.html. Sept 2014.
Acknowledgements
This work was supported by the Slovak Grant Agency for Science under the grant VEGA 1/0006/12 and by the Slovak Research and Development Agency Project ID: APVV-0487-11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chromčíkova, M., Liška, M., Zemanová, V. et al. Thermodynamic model and Raman spectra of CaO–P2O5 glasses. J Therm Anal Calorim 121, 269–274 (2015). https://doi.org/10.1007/s10973-015-4515-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4515-2