Abstract
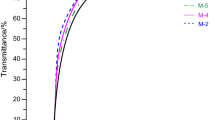
New segmented poly(thiourethane-urethane)s (PTU-Us) (with hard-segment content of 30–60 mass%) were synthesized by a one-step melt polymerization from poly(oxytetramethylene) diol of \( \overline{M}_{n} \) = 1,000 g mol−1 or \( \overline{M}_{n} \) = 2,000 g mol−1 or poly(hexamethylene carbonate) diol of \( \overline{M}_{n} \) = 860 g mol−1 as soft segments, 1,1′-methanediylbis(4-isocyanatocyclohexane) (Desmodur W ®) and (methylenedi-1,4-phenylene)dimethanethiol as a chain extender. The PTU-Us were examined by FTIR, GPC, XRD, DSC, TG, Shore hardness, and tensile testing. Moreover, refractive index, transparency, adhesive properties, and resistance to bacteria and fungi were determined for selected polymers. The obtained high-molar-mass amorphous polymers showed elastomeric or plastic properties. Their T gs were in the range from −70 to 58 °C. The PTU-Us with the polycarbonate soft segments demonstrated a better segmental miscibility (higher T gs), transparency as well as generally higher tensile strength and hardness than those with the polyether soft segments. All the synthesized PTU-Us showed a relatively good thermal stability. The temperature of 1 % mass loss of all PTU-Us was in the range of 236–255 °C. The introduction of thiourethane linkages to polyurethane chain caused increase of the adhesive strength on copper–polymer junction and refractive index values. From the microbial studies, it was found that the obtained polymers had delayed the growth of Gram-positive bacteria.












Similar content being viewed by others
References
Kultys A. Sulfur-containing polymers. Encyclopedia of polymers science and technology. 2010. (http://onlinelibrary.wiley.com/doi/10.1002/0471440264.pst355.pub2).
Jha GS, Seshadri G, Mohan A, Khandal RK. Sulfur containing optical plastics and its ophthalmic lenses applications. e-Polymers. 2008;035:1–27.
Tyagi M, Suri G, Chhabra P, Seshadri G, Malik A, Aggarwal S, Khandal RK. Novel way of making high refractive index plastics; metal containing polymers for optical applications. e-Polymers 2009; 100.
Ioan S, Macocinschi D, Filip D, Taranu A. Specific refractive index increments of segmented poly(ester urethanes)s. Polym Test. 2002;21:757–62.
Hirano H, Kadota J, Agari Y, Harada T, Tanaka M, Hasegawa K. Linear polymers with sulfur in the main chain. IV. Synthesis of thermotropic liquid–crystalline polythioesters based on 4,4′-biphenyldithiol with excellent adhesive properties. Polym Eng Sci. 2007;47:262–9.
Talarico LB, Pujol CA, Zibetti RGM, Faria PCS, Noseda MD, Duarte MER, Damonte EB. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir Res. 2005;66(2–3):103–10.
De Clercq E. New anti-HIV agents and targets. Med Res Rev. 2002;22(6):531–65.
Vietti D. Polysulfides. Encyclopedia of polymers science and technology, vol. 3. New Jersey: Wiley; 2003. p. 732–44.
El-Hibri MJ, Weineberg SA. Polysulfones. Encyclopedia of polymers science and technology, vol. 4. New Jersey: Wiley; 2003. p. 1–25.
Kricheldorf HR, Schwarz G. Poly(thioester)s. J Macromol Sci A Pure Appl Chem. 2007;44:625–49.
Li Q, Zhou H, Wicks DA, Hoyle CE, Magers DH, McAlexander HR. Comparison of small molecule and polymeric urethanes, thiourethanes, and dithiourethanes: hydrogen bonding and thermal, physical, and mechanical properties. Macromolecules. 2009;42:1824–33.
Lipatov YS, Muller S, Privalko VP, Singer H. Untersuchung der thermischen eigenschaften von polythiourethan-elastomeren auf der basis von oligobutadien. Angew Makromol Chem. 1979;82:79–87.
Shin J, Matsushima H, Chan JW, Hoyle CE. Segmented polythiourethane elastomers through sequential thiol-ene and thiol-isocyanate reactions. Macromolecules. 2009;42:3294–301.
Gorna K, Polowinski S, Gogolewski S. Synthesis and characterization of biodegradable poly(ε-caprolactone urethane)s. I. Effect of the polyol molecular weight, catalyst, and chain extender on the molecular and physical characteristics. J Polym Sci A Polym Chem. 2002;40:156–70.
Gorna K, Gogolewski S. In vitro degradation of novel medical biodegradable aliphatic polyurethanes based on ε-caprolactone and pluronics1 with various hydrophilicities. Polym Degrad Stab. 2002;75:113–22.
Eglin D, Griffon S, Alini M. Thiol-containing degradable poly(thiourethane-urethane)s for tissue engineering. J Biomas Sci Polym Ed. 2010;21:477–91.
Kultys A, Rogulska M, Pikus S. The synthesis and characterization of new thermoplastic poly(thiourethane-urethane)s. J Polym Sci A Polym Chem. 2008;46:1770–82.
Kultys A, Rogulska M, Olszewska E. New thermoplastic poly(thiourethane-urethane) elastomers based on hexane-1,6-diyl diisocyanate (HDI). J Therm Anal Calorim. 2013;114(2):903–16.
Wirpsza Z. Polyurethanes: chemistry, technology and applications. New York: Ellis Horwood; 1993.
Hepburn C. Trends in polyurethane elastomer technology. Iran J Polym Sci Technol. 1992;1(2):84–110.
Jaffrennou B, Droger N, Méchin F, Halary JL, Pascault JP. Characterization, structural transitions and properties of a tightly crosslinked polythiourethane network for optical applications. e-Polymers 2005;082.
Podkoscielny W, Podgorski M. Linear polythioesters. 26. Products of interfacial polycondenation of bis(4-mercaptomethylphenyl)methane with some aliphatic and isomeric phthaloyl dichlorides. Angew Makromol Chem. 1996;241:135–48.
Dispersity in polymer science (IUPAC Recommendations 2009) prepared for publication by R. F. T. Stepto. Pure Appl Chem 2009;81(2):351–3.
Rabiej M, Rabiej S. Analysis of X-ray diffraction pattern of polymers by means of WAXSFIT computer program (in Polish). Poland: ATM; 2006.
Lee DK, Tsai HB, Tsai RS. Preparation and properties of transparent thermoplastic segmented polyurethanes derived from different polyols. Polym Eng Sci. 2007;47(5):695–701.
Chen PH, Yang YF, Lee DK, et al. Synthesis and properties of transparent thermoplastic segmented polyurethanes. Adv Polym Technol. 2007;26(1):33–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kultys, A., Puszka, A. Transparent poly(thiourethane-urethane)s based on dithiol chain extender. J Therm Anal Calorim 117, 1427–1439 (2014). https://doi.org/10.1007/s10973-014-3877-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3877-1