Abstract
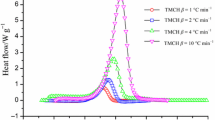
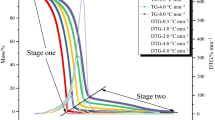
Possessing thermal instability inherently, organic peroxides have caused many severe accidents in chemical industries all over the world. tert-Butyl hydroperoxide (TBHP) is usually used as initiator or oxidant because of its strong oxidizing ability in the chemical process. In this study, the thermal hazard analysis of TBHP mixed with various acids was investigated. Differential scanning calorimetry (DSC) and vent sizing package 2 were used to figure out the thermal runaway behaviors of TBHP. Thermokinetic parameters, such as exothermic onset temperature (T 0), maximum temperature (T max), and enthalpy (ΔH), were obtained from thermal curves. In addition, the activation energy (E a) and rate constant (k) were calculated by the Arrhenius equation. Therefore, the T 0 was determined to be 91.6 °C for exothermic reaction using DSC under 4 °C min−1 of heating rate. The E a for exothermic reaction was calculated to be 92.38 kJ mol−1 by DSC in this study. As far as loss prevention is concerned, thermokinetic parameters are crucial to the relevant processes in the chemical industries, particularly under process upsets.



Similar content being viewed by others
References
Liu SH, Lin CP, Shu CM. Thermokinetic parameters and thermal hazard evaluation for three organic peroxides by DSC and TAM III. J Therm Anal Calorim. 2011;106:165–72.
Fisher HG, Goetz DD. Determination of self-accelerating decomposition temperatures for self-reactive substances. J Loss Prev Process Ind. 1993;6(3):305–16.
Wu KW, Hou HY, Shu CM. Thermal phenomena studies for dicumyl peroxide at various concentrations by DSC. J Therm Anal Calorim. 2006;83:41–4.
You ML, Tseng JM, Liu MY, Shu CM. Runaway reaction of lauroyl peroxide with nitric acid by DSC. J Therm Anal Calorim. 2010;102:535–9.
Yeh PY, Shu CM, Duh YS. Thermal hazard analysis of methyl ethyl ketone peroxide. Ind Eng Chem Res. 2003;42(1):1–5.
Chang RH, Tseng JM, Jehng JM, Shu CM, Hou HY. Thermokinetic model simulations for methyl ethyl ketone peroxide contaminated with H2SO4 or NaOH by DSC and VSP2. J Therm Anal Calorim. 2006;83:57–62.
Tseng JM, Chang RH, Horng JJ, Chang MK, Shu CM. Thermal hazard evaluation for methyl ethyl ketone peroxide mixed with inorganic acids. J Therm Anal Calorim. 2006;85:189–94.
Tseng JM, Chang YY, Su TS, Shu CM. Study of thermal decomposition of methyl ethyl ketone peroxide using DSC and simulation. J Hazard Mater. 2007;142:765–70.
Shen SJ, Wu SH, Chi JH, Wang YW, Shu CM. Thermal explosion simulation and incompatible reaction of dicumyl peroxide by calorimetric technique. J Therm Anal Calorim. 2010;102:569–77.
Chen JR, Wu SH, Lin SY, Hou HY, Shu CM. Utilization of microcalorimetry for an assessment of the potential for a runaway decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2008;93:127–33.
Chu YC, Chen JR, Tseng JM, Tsai LC, Shu CM. Evaluation of runaway thermal reactions of di-tert-butyl peroxide employing calorimetric approaches. J Therm Anal Calorim. 2011;106:227–34.
Liang YC, Jhu CY, Wu SH, Shen SJ, Shu CM. Evaluation of adiabatic runaway reaction of methyl ethyl ketone peroxide by DSC and VSP2. J Therm Anal Calorim. 2011;106:173–7.
Wang YW, Shu CM, Duh YS, Kao CS. Thermal runaway hazards of cumene hydroperoxide with contaminants. Ind Eng Chem Res. 2001;40:1125–32.
Wang YW, Duh YS, Shu CM. Thermal runaway hazards of tert-butyl hydroperoxide. J Therm Anal Calorim. 2009;95(2):553–7.
National Fire Protection Association. Code for the Storage of Organic Peroxide Formulations, NFPA 432. USA. 2002.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Vincent L, Sbirrazzuoli N, Vyazovkin S. Evaluation of the dynamic response of a new heat flux calorimeter for kinetic purposes. Ind Eng Chem Res. 2002;41:6650–5.
Wang YW, Duh YS, Shu CM. Evaluation of adiabatic runaway reaction and vent sizing for emergency relief from DSC calorimetry. J Therm Anal Calorim. 2006;85:225–34.
Ando T, Fujimoto T, Morisaki S. Analysis of differential scanning calorimetric data for reactive chemicals. J Hazard Mater. 1991;28:251–80.
Maria G, Heinzle E. Kinetic system identification by using short-cut techniques in early safety assessment of chemical processes. J Loss Prev Process Ind. 1998;11:187–206.
STARe Software with Solaris Operating System. Operating Instructions. Zurich: Mettler Toledo; 2012.
VSP2 manual and methodology. Fauske and Associates. Burr Ridge: LLC; 2012.
Wang YW, Shu CM, Duh YS, Kao CS. Thermal runaway hazards of cumene hydroperoxide with contaminants. Ind Eng Chem Res. 2001;40:1125–32.
Andreozzi R, Caprio V, Crescitblli S, Russo G. The thermal stability of tert-butyl hydroperoxide-acid mixtures. J Hazard Mater. 1988;17:305–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chou, HC., Chen, NC., Hsu, ST. et al. Thermal hazard evaluation of tert-butyl hydroperoxide mixed with four acids using calorimetric approaches. J Therm Anal Calorim 117, 851–855 (2014). https://doi.org/10.1007/s10973-014-3777-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3777-4