Abstract
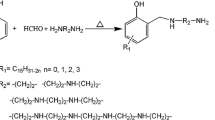
Phenyl bisthioureas: 4,4′-(bisthiourea)diphenylmethane (DTM), 4,4′-(bisthiourea)diphenyl ether (DTE), and 4,4′-(bisthiourea)diphenyl sulfone (DTS) were synthesized and used as curing agents for the epoxy resin diglydicyl ether bisphenol A (DGEBA). Synthesized phenyl bisthioureas were characterized using FT-IR and 1H-NMR analysis. For comparison studies the epoxy system was also cured using the conventional aromatic amine 4,4′-diaminodiphenyl ether (DDE). Curing kinetics of epoxy/amine system was studied by dynamic and isothermal differential scanning calorimeter (DSC). Curing kinetic was evaluated based on model-free kinetics (MFK) and ASTM E 698 model, and the activation energy was compared with DDE. Curing system of phenyl bisthiourea link (DGEBA/DTM, DGEBA/DTE, and DGEBA/DTS) shows two exothermic peaks, while that of the conventional aromatic amines showed only a single peak. The initial exothermic peak is due to the primary nitrogen of the thiourea group, and the exotherm at higher temperature is due to the presence of thiourea groups. Glass transition temperature (T g) of DGEBA/DTM, DGEBA/DTE, and DGEBA/DTS cured resins were lowered by 323 K when compared to the widely used diaminodiphenyl ether (DDE) cured resin. Oxidation induction temperature measurement performed on DSC suggests that the DGEBA/DTM, DGEBA/DTE, and DGEBA/DTS system cured resins has better oxidative stability when compared to cured DGEBA/DDE resin system.













Similar content being viewed by others
References
Rwei SP, Cheng CY, Lioue GS, Cheng KC. Curing and pyrolysis of cresol novolac epoxy resins containing BABODOPN. Polym Eng Sci. 2005;45:478–86.
Wang CS, Lee MC. Synthesis and properties of epoxy resins containing 2-(6-oxid-6H-dibenz(c,e)(1,2)oxaphosphorin-6-yl) 1,4-benzenediol(II). Polymer. 2000;41:3631–8.
Pan G, Du Z, Zhang C, Li C, Yang X, Li H. Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety. Polymer. 2007;48:3686–92.
Gu X, Wei H, Huang X, Tang X. Synthesis and characterization of a novel curing agent for epoxy resin based on phosphazene derivatives. J Macromol Sci A Pure Appl Chem. 2010;47:828–32.
Liu H, Xu K, Al H, Chen M. Preparation and characterization of phosphorus-containing Mannich-type bases as curing agents for epoxy resin. Polym Adv Technol. 2009;20:753–8.
Tseng F, Chang F, Lin S, Liu J. Preparation epoxy curing of novel dicyclopentadiene-derived Mannich amines. J Appl Polym Sci. 1999;71:2129–39.
Lin J, Lin S, Kuo T, Chang F, Tseng F. Synthesis and epoxy curing of Mannich bases derived from bisphenol-A and poly(oxyethylene) diamine. J Appl Polym Sci. 2000;78:615–23.
Liu J, Zang X, Zhang X, Xia X. Synthesis and curing properties of novel curing agent based on N-(4-hydroxyphenyl) maleimide and dicyclopentadiene moieties. J Appl Polym Sci. 2011;120:56–61.
Santosh KR, Ambroise D, Zoubida S, Freddy YC, Marc JM. The effect of amines on the UV-curing of epoxy resins. Iran Polym J. 2006;15:855–62.
Zang D, Jia D, Chen S. Kinetics of curing and thermal degradation of hyperbranched epoxy (HTDE)/diglycidyl ether of bisphenol-A epocy hybrid resin. J Therm Anal Calorim. 2009;98:819–24.
Supriya N, Catherine KB, Rajeev R. DSC-TG studies on kinetics of curing and thermal decomposition of epoxy-ether amine systems. J Therm Anal Calorim. 2010;101:1011–7.
Wan J, Li C, Bu Z-Y, Fan H, Li B-G. Evaluating a four-directional benzene-centered aliphatic polyamine curing agent for epoxy resins. J Therm Anal Calorim. 2012. doi:10.1007/s10973-012-2863-8.
Cruz JD, Venkatachalam TK, Uekun FM. Novel thiourea compounds as dual-function microbicides. Biol Reprod. 2000;63:196–205.
Vasanthi BJ, Ravikumar L, Selvaraj A. Corrosion control of phenylthiourea polymers on aluminium in alkaline medium. Mater Corros. 2008;59:14–20.
Sabaa MW, Mohamed RR, Yassin AA. Organic thermal stabilizers for rigid poly(vinyl chloride) VII. Phenylurea and phenylthiourea derivatives. Polym Degrad Stab. 2008;81:37–45.
Vasanthi BJ, Ravikumar L. Synthesis and characterization of new poly(azomethine ester)s having phenylthiourea units. Eur Polym J. 2007;43:4325–31.
Ravi Kumar L, Sengodan V, Balaji Prasad M, Gopalakrishnan K, Sethupathi K. Synthesis and characterization of conductive blends of polyaniline with poly(azomethine ester)s. Int J Polym Mater. 2007;56:197–206.
Ramesh P, Ravikumar L, Burkanudeen AR. Synthesis and characterization of epoxy-containing Schiff-base and phenylthiourea groups for improved thermal conductivity. Polym Plast Technol Eng. 2012;51:140–5.
Haimei W, Yuechao Z, Lirong Z, Zongjie D, Baolong Z, Yuying Z. Curing behaviors and kinetic of epoxy resins with a series of biphenyl curing agents having different methylene units. Thermochim Acta. 2011;521:18–25.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–6.
Prime RB. Relationship between isothermal and dynamic cure of thermosets via the isoconversion representation. Polym Eng Sci. 1973;13:365–72.
Schmid M, Ritter A, Affolter S. Determination of oxidation induction time and temperature by DSC results of round robin tests. J Therm Anal Calorim. 2006;83:367–71.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh, P., Ravikumar, L. & Burkanudeen, A. Curing properties of novel curing agent based on phenyl bisthiourea for an epoxy resin system. J Therm Anal Calorim 115, 713–722 (2014). https://doi.org/10.1007/s10973-013-3309-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3309-7