Abstract
We show, through thermal conductivity coefficient measurements of Li2TiGeO5 ceramics, that the anomaly associated with the ferroelastic phase transition can be distinguished. We correlate this anomaly with the measurement of the domain structure observed in the vicinity of phase transition temperature T c. Near the T c, the appearance of ferroelastic domains which become new scattering centers for thermal waves, strongly affect the thermal conductivity in Li2TiGeO5 ceramics.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Lithium titanium germanate (Li2TiGeO5) belongs to the class of materials of the general formula A2TiMO5 (where A = Li, Na; and M = Si, Ge) with the characteristic name natisite, which is derived from the naturally occurring mineral in nature Na2TiSiO5 [1]. Li2TiGeO5 was first synthesized in 1999 [2] and its ferroelastic properties have been reported by Poprawski et al. [3]. Li2TiGeO5 may be considered as a layered structure with alternating layers of TiO5 pyramids sharing the basal O(2) atoms with GeO4 tetrahedra, and cationic layers of Li+. The neighboring pyramids are related by n-glide planes in such a way that the titanyl Ti–O short bonds are antiparallel in adjacent rows of the polyhedral layer [4, 5]. A2TiMO5 crystals exhibit ferroelastic properties and very small value of the thermal expansion coefficient, especially in paraelastic phase [6, 7]. This property allows to use these materials as a matrix for high power solid state lasers where the thermal stability plays a crucial role in the construction.
Ferroelastic ceramics are composed of many crystallites with crystallographic orientations changing from one to another along the grain boundaries. When temperature decreases below the Curie temperature T c, a spontaneous deformation and formation of ferroelastic domains take place simultaneously in each of the grains. The ferroeleastic properties of ceramics result in unusual physical properties of these materials [8]. Such a big interest in ceramics is due to the fact that these materials are relatively easy to manufacture and offer a wide range of easily accessible modifications in contrast to single crystals.
Ceramics produced in the sintering process do not have any limitations as for the orientation of grains and their distribution, while preserving a good single crystal structure [9]. This freedom of grain orientation can improve the properties of ferroelastic material [10].
Widely used thermal conductivity measurements allow for the analysis of the lattice vibrations and in consequence, to describe the phase transition [11, 12]. It is well known that in these experiments with ferroelastic ceramics, especially at low-temperature phase, the phonon analysis is hindered by a strong scattering at grain boundaries and domain walls [13]. Many experimental results of heat transport in ferroelectric and ferroelastic crystals showed nontrivial nature of anomalies associated with phase transition and domain structure in these materials [14, 15].
The aim of this work is experimental verification how the appearance of ferroelastic domain influences the thermal conductivity of Li2TiGeO5 ceramics. In the critical region of phase transition, the domain structure formation may strongly affect the heat transport, and for this reason, an anomaly of thermal conductivity coefficient is expected.
Experimental
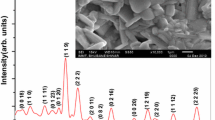
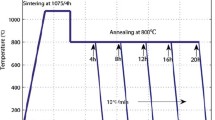
For the synthesis of Li2TiGeO5 ceramics the following reagents were used: lithium carbonate (Li2CO3), titanium oxide (TiO2), and germanium oxide (GeO2). The reactants were thoroughly mixed in a stoichiometric proportions in a mill for about 30 min. Then, from the resulting mixture, the pellet was formed and sintered in a few stages. Initially, it was sintered at 1070 K for 12 h, which was followed by cooling to room temperature. The resulting sinter was crushed and re-formed into a pellet to a final sintering at 1240 K for 12 h. At the end, the ceramic was slowly cooled down to room temperature at a rate of 50 mK min−1. The average grain size was estimated from the X-ray diffractograms, it amounts to about few microns. For thermal conductivity measurements, the rectangular samples (3 × 3 × 8 mm) of Li2TiGeO5 ceramics were prepared.
The chamber for thermal conductivity coefficient measurements was isolated by four screens, the temperature inside was controlled using platinum and germanium thermometers. The temperature stabilization was kept on ±3mK. The sample temperature was measured with a constantan–manganin thermocouple. The longitudinal temperature gradient was induced by a small electric heater, attached to the sample. The obtained temperature gradients were in the range of 0.2–0.3 K. To reduce the radiative conductance and heat losses the insulation shields have been used, and the whole measurement was performed in a high vacuum. The temperature distribution on the deepest shield was maintained on a similar level as on the sample. The maximum standard deviation of less than ±5 % was obtained (mainly caused by inaccuracy of the geometry of the specimen) and the standard deviation obtained from the dispersion of the measurement points did not exceed 2 %.
Results and discussion
The temperature dependence of the thermal conductivity coefficient of Li2TiGeO5 ceramics in the temperature range 80–350 K is presented in Fig. 1. One can distinguish three characteristic regions in the considered temperature range. Starting from the lowest temperature, the thermal conductivity coefficient is well described by the relation κ ~1/T which results dominantly from the phonon–phonon scattering (inset in Fig. 1) [16]. This relation is perturbed at 160 K where the thermal conductivity slightly starts to increase with temperature. Then, the region close to 200 K can be considered as a minimum, because further temperature increase causes an increase in thermal conductivity. It evidently stops to increase at T = 235 K which corresponds to the ferroelastic phase transition temperature.
In bulk material, the thermal conductivity of the dielectric is determined by the crystal lattice vibrations whose quanta are well known as phonons. All imperfections of crystal structure like defects, domain structure, especially ferroelastic one etc. perturb the heat flow through the sample by changing the mean free path of phonons. It is worth emphasizing that the ferroelastic phase transition is accompanied by the symmetry change, which involves a modification of dynamics of the crystal lattice [17] and as a consequence leads to the changes in the thermal conductivity coefficient. The observed characteristic minimum of the thermal conductivity coefficient in Li2TiGeO5 is mainly due to the emergence of domain structure and the following changes. It should be remembered that the investigated material is a ceramic, polycrystalline material, where the phonons are scattered by the inner grain boundaries because of the elastic mismatch on them [13]. Thus, the observed changes in thermal conductivity are superposition of the lattice dynamics, the domain structure changes and the grain size effect. However, the grain size effect play a crucial role in low temperatures [18], hence, the domain structure effect become dominant in the investigated temperature range. The influence of the ferroelastic domain structure appearance on thermal properties was recently observed in Cs x (NH3)1−x LiSO4 crystal [19].
In order to verify the claim thesis that the change in thermal conductivity below 235 K is connected with the emergence of ferroelastic domain structure, we analyzed how the domain structure was changing in the investigated temperature range. For this purpose, the domain structure observation of Li2TiGeO5 crystal was performed, which we made by the spontaneous birefringence imaging measurements [20]. Photos from the polarizing microscope at selected temperatures, showing the changes in the domain structure are shown in Fig. 2. In paraelastic phase, where no ferroelastic domains exist, the thermal conductivity does not show any anomalies. During the temperature lowering, the appearance of optically inhomogeneous regions indicating the formation of the domain structure begins at T = 235 ± 1 K. The change of the Li2TiGeO5 crystal symmetry during the structural phase transition according to Aizu’s notation 4/mmmFmmm, leads to ferroelastic domains occurrence of two types [21]. The domains are separated by mutually perpendicular domain walls of two types W a and W b which make an angle 45° with respect to the (100) and (010) crystallographic axes. This is in accordance with the theoretical predictions of ferroelastic domain walls in orthorhombic phase: y = ±x [22]. When the temperature decreases, the number of domain walls in the form of narrow perpendicular strips rapidly increases, which means the increase of the number of the scattering centers, and as a result, the thermal conductivity decreases. At further temperature decrease, the saturation of the domain walls appearance occurs, which in the measurement of the thermal conductivity coefficient is observed as a characteristic minimum. Then, the grain size effect starts to play a decisive role in the transport of heat until the phonon–phonon scattering becomes dominant. It can be seen that, apart from the phase transition temperature (150 K), the number of domain walls becomes unchanged. A comparison of the ferroelastic domain structure at 150 and 200 K is presented in Fig. 2. It should be point out here that the observed mechanism of thermal conductivity is reversible with temperature during cooling and heating.
Conclusions
Measurements of thermal conductivity of Li2TiGeO5 ceramics revealed anomalous changes in thermal properties below ferroelastic phase transition temperature. The distinct deviation from values of the paraelastic thermal conductivity is attributed to the elastic domain formation. The domain structure formation observed with the polarizing microscope unequivocally confirmed that the increase in the number of domain walls, which become new scattering centers for phonons, strongly affect the thermal conductivity in Li2TiGeO5 ceramics.
References
Nyman H, O’Keeffe M, Bovin JO. Sodium titanium silicates NaTiSiO5. Acta Crystallogr B. 1978;34:905–6.
Bastow TJ, Botton GA, Etheridge J, Smith ME, Whitfield HJ. A study of Li2TiOSiO4 and Li2TiOGeO4 by X-ray powder and electron single-crystal diffraction. Acta Crystallogr A. 1999;55:127–32.
Poprawski R, Przeslawski J, Kireev VV, Shaldin YuV, Just M. Ferroelastic phase transition in Li2TiGeO5 crystals. Phys Status Solidi A. 2001;183:R7–9.
Sieradzki A, Poprawski R, Pietraszko A. Investigation of the crystal structure and influence of hydrostatic pressure on phase transition in Li2TiGeO5 ferroelastics. Phase Transitions. 2004;77:289–94.
Kireev VV, Yakubovich OY, Ivanov-Shits AK, Mel’nikov OK, Dem’yanets LN, Skunman J, Chaban NG. Growth, structure and electrophysical properties of single crystals of A2TiGeO5 (A = Li and Na). Russ J Coord Chem. 2001;27:31–7.
Przesławski J, Poprawski R, Just M, Kireev VV, Mielcarek S, Mróz B. Li2TiGeO5: a novel ferroelastic crystal. Ferroelectrics. 2002;267:201–8.
Sieradzki A, Szewczyk D, Nankiewicz M, Jeżowski A, Poprawski R, Ciżman A. Evidence of the ferroelastic phase transition in Na2TiGeO5 ceramics. Phase Transitions. 2013;86:301–5.
Jones JL, Hoffman M. R-curve and stress–strain behavior of ferroelastic ceramics. J Am Ceram Soc. 2006;89:3721–7.
Vullum PE, Mastinm J, Wright J, Einarsrud M-A, Holmestad R, Grande T. In situ synchrotron X-ray diffraction of ferroelastic La0.8Ca0.2CoO3 ceramics during uniaxial compression. Acta Mater. 2006;54:2615–24.
Jones JL, Hoffman M, Bowman KJ. Saturated domain switching textures and strains in ferroelastic ceramics. J Appl Phys. 2005;98:024115.
Tachibana M, Kolodiazhnyi T, Takayama-Muromach E. Thermal conductivity of perovskite ferroelectrics. Appl Phys Lett. 2008;93:092902.
Jeżowski A, Poprawski R, Mróz J. Thermal conductivity anomalie in (CH3NH3)5Bi2Br11 ferroelectric crystals. J Korea Phys Soc. 1988;32:S210.
Mielcarek S, Mróz B, Tylczyński Z, Piskunowicz P, Trybuła Z, Bromberek M. Low-temperature thermal conductivity of ferroelastic Gd2(MoO4)3. Phys B. 2001;299:83–7.
Strukov BA, Belov AA. Heat transport properties and related materials. Phase Transitions. 1994;51:175–97.
Jendoubi H, Hellali D, Zamali H, Jemal M. The phase diagram of KNO3–RbNO3. J Therm Anal Calorim. 2013;111:877–83.
Berman R. Thermal conduction in solids. Oxford: Clarendon Press; 1976.
Salje EKM. Phase transitions in ferrroelastic and co-elastic crystals. Cambridge: Cambridge University Press; 1990.
Rice RW. Mechanical properties of ceramics and composites grain and particle effects. New York: Marcel Dekker; 2003.
Czaja P. Detection of a ferroelastic phase transition in Cs x (NH4)1-x LiSO4 with the use of the DSC method. J Therm Anal Calorim. 2013;. doi:10.1007/s10973-013-2942-5.
Sieradzki A, Ciżman A, Poprawski R, Shuvaeva V, Glazer AM. Birefringence imaging of phase transition in ferroelastic Li2TiGeO5. Phase Transition. 2005;78:351.
Sapriel J. Domain-wall orientations in ferroelastics. Phys Rev B. 1975;12:5128–40.
Aizu K. Determination of the state parameters and formulation of spontaneous strain for ferroelastic. J Phys Soc Jpn. 1970;28:706–16.
Acknowledgments
The present work has been financially supported by the Polish Ministry of Scientific Research and Information Technology, Department of Scientific Research (Grant No. N507 481388).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sieradzki, A., Jeżowski, A. & Poprawski, R. The influence of ferroelastic domain formation on thermal conductivity in Li2TiGeO5 ceramics. J Therm Anal Calorim 115, 467–470 (2014). https://doi.org/10.1007/s10973-013-3279-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3279-9