Abstract
The composite anode material for solid oxide fuel cell (SOFC) based on Ni/3YSZ (where 3YSZ means 3 mol% Y2O3 in ZrO2) has been prepared (by means of modified citric method) and studied (structure, microstructure and electrical properties). In order to improve ionic conductivity of 3YSZ, Al2O3 was added at a synthesis stage to cermet material. The application of co-precipitation synthesis method results in obtaining uniform, nanometric powders. The methods of thermal analysis (TG/DTA/EGA and dilatometry) were used to determine thermal treatment of samples and to study mechanical compatibility of electrolyte and cermet anode material. The results of electrical properties measurements show that the addition of small amount of Al2O3 to 3YSZ causes its conductivity improvement and this effect is strongest for the smallest amount of Al2O3.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Current activities in the development of SOFC are concentrated on decreasing of operating temperature of cells, reducing costs and improving thermochemical stability of components. From the point of view of anode of the SOFC, the most important properties taken into account are high mixed electronic-ionic conductivity, sufficient electrocatalytic activity in reaction of fuel oxidation, chemical stability and thermal compatibility with electrolyte material. Ni/YSZ cermet is an electrode material used and improved for many years [1]. Cubic 8YSZ (8 mol% Y2O3 in ZrO2) has been commonly used in high temperature SOFC due to high oxide ionic conductivity, but it reveals poor resistance to thermal shock and low mechanical strength. On the other hand, tetragonal zirconia 3YSZ ensures superior mechanical properties. Unfortunately, this particular form has two times lower conductivity than 8YSZ at high temperatures (ca. 1,000 °C). As it has been shown by Ghatee et al. [2], the addition of 3YSZ material to 8YSZ results in decrease of electrical conductivity with simultaneous improvement of toughness and fracture strength of composite above 550 °C. Incorporation of some dopants can improve properties of YSZ materials. It is widely known that the addition of small amount of alumina to zirconia decreases sintering temperature of zirconia materials [3–5]. What is more, the addition of Al2O3 to 3YSZ can improve electrical properties by grain boundary modification [6, 7]. Electrocatalytic activity is directly connected with three-phase boundary (TPB) area consisted of electronic conductor (metal), oxygen ionic conductor (YSZ) and gas phase. Nickel particles act as an electronic conductor (increasing the overall electronic conductivity) and catalyst in oxidation reactions. To obtained nanometric and homogenous materials, several different wet preparation methods such as citric combustion [8], gel–combustion with glycine [9], nitrate with urea combustion [10] or co-precipitation with NaOH methods [11] can be used. This type of microstructure ensures developing of TPB.
Experimental
The modified citric method was used in order to obtain two series of samples with different composition: first one, 3YSZ—3 mol% yttria-stabilised zirconia samples containing Al2O3 in the amount—0.5, 1.0 or 2.0 mass% (labelled as Al2O3–3YSZ), and the second one, where the same material but with the addition of nickel with mass ratio of Ni to Al2O3–3YSZ fixed to 1:1 was co-precipitated (labelled as Ni/Al2O3–3YSZ).
The saturated solutions of yttrium, zirconyl, aluminium and nickel (in case of Ni/Al2O3–3YSZ synthesis) nitrates were prepared and mixed in proper ratio in order to obtained Al2O3–3YSZ and 50 %Ni/Al2O3–3YSZ materials. All reagents used were analytically pure (provided by Sigma–Aldrich) and their compositions were analytically verified by classical chemical analysis (mass method). Next, the calculated amount of citric acid monohydrate (with 5 mol% excess) was added to the solution consisting of mixed nitrates. The resulting solution was left for a few hours on a hot plate (around 200 ± 5 °C) to evaporate and then was stirred. The final solution turned to grey-green gel, which afterwards was heated on the burner. Then disc pellets were pressed and sintered at 800 and 1,200 °C in air atmosphere. Before dilatometric and impedance measurements, the pellets were reduced at 800 °C by 10 %H2/90 %Ar mixture.
Apparatus
XRD analysis was used to determine the phase composition of the obtained materials. The analysis was performed on Philips X’Pert Pro diffractometer with monochromatized CuKα radiation, within the range of diffraction angles, 2θ from 10° to 80°. Crystallite sizes were calculated from the X-ray line broadening of the selected peaks [(011) for ZrO2 and (002) for NiO] by using the Scherrer approach and diffractometer software.
The calcination temperatures and thermal expansion coefficients (TEC) were determined by thermal analysis methods. TG/DTA measurements with gaseous analysis (EGA) were carried out by means of SDT 2960 TA Instruments apparatus with mass spectrometer (Balzers, ThermoStar QMD 300) connected online. All tests were performed in synthetic air conditions with temperature change rate set to 10 deg min−1. The gas flow rate was set to 0.1 dm3 min−1 and the samples with mass of about 10 mg within the platinum crucibles were placed. The empty platinum crucibles were used as reference ones.
Dilatometric studies were performed by means of measuring instrument and displacement transducer provided by DIL 402 C equipment from NETZSCH. The measurements were carried out in the mixture of 5 %H2 in Ar; a rate of temperature change was set to 5 deg min−1 within the temperature range from 20 to 700 °C. Cuboidal samples of dimensions 2 × 2 × 9 mm3 were used in the experiments. The coefficients of thermal expansion were calculated for all samples, using the linear regression approximation.
The microstructure observations were carried out using JROL 5400 scanning electron microscope equipped with energy dispersive X-ray analysing system (EDX).
The electrical properties were investigated by ac-electrochemical impedance spectroscopy. The measurements were carried out by means of Solatron SI 1260 Impedance/Gain-Phase Analyzer with the SI 1296 dielectric interface. The temperature and frequency range were set to 20–750 °C and 0.1–106 Hz, respectively, and a mixture of 10 %H2 in Ar was used as a gas atmosphere.
Results and discussion
The results of thermal measurements TG/DTA with analysis of gaseous products (EGA) of obtained dried gel (Fig. 1) show that a big loss of mass started above 200 °C with accompanying exothermic DTA pick (with maximum around 360 °C). Exothermic character of curves demonstrates that decomposition is connected with simultaneous oxidation processes. The mass spectrum shows evolving of water and carbon dioxide in temperature range corresponding to DTA pick while the ionic current originating from NOx remains at the same level, which proves that the procedure used for dried gel formation was sufficient for nitrates decomposition. On the other hand, the temperature below 220 °C was too low for decomposition of citrates to take place (decomposition of these compounds is completed above 400 °C). According to Banerjee et al. [12], citrate–nitrate gel, precursor of ZrO2, decomposes at 600 °C (the maximum of pick at 570°) which is around 200 °C higher than presented in this paper. It is the most probable that materials obtained in this work consisted of much smaller particles then Banerjee materials, which result in decreasing of decomposition temperature. Wyrzykowski et al. [13] presented TG/DTA curves for citric acid decomposition and according to these authors it finally decomposes at around 270 °C. A slight excess of citric acid which was introduced by us to nitrate mixture decomposed below this temperature.
After calcination of materials, the TG/DTA measurements were performed once again (Fig. 2). The small mass loss below 200 °C corresponds to water desorption. Effect on TG curve is confirmed by decrease of ionic current of water. Above 700 °C, slow mass increase is observed and in the temperature range 700–810 °C the overall mass increase was equal to 0.123 ± 0.001 mass% with respect to initial sample mass. This is probably the result of oxygen incorporation in the non-stoichiometric oxides structures. The same effect was observed earlier by other authors [14].
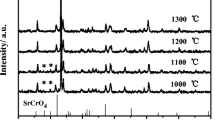
X-ray analysis of materials obtained after calcinations (Fig. 3) confirmed that the only phases present in synthesised materials were tetragonal ZrO2 and cubic nickel oxide (in case of sample with 50 mass% of nickel). The identification of Al2O3 is not possible by this method due to insufficient amount of aluminium oxide in materials. In case of materials with nickel (Ni/Al2O3–3YSZ) obtained via sol–gel synthesis there is a chance of NiAl2O4 formation around 800–900 °C [15, 16], while for materials obtained by solid state reaction this spinel is formed in much higher temperatures [17]. As it was shown in the literature [18] for alumina content equal to 1 mass% of the Ni/YSZ, the diffraction pattern for NiAl2O4 appears. The authors obtained the material by solid state reaction in 1,350 °C. The amount of alumina equal to 1 mass% in cited paper corresponds to 2 mass% content in our work. In our material, however, no diffraction pattern characteristic for NiAl2O4 was observed, which suggests that most probably the modified citric method at such low temperature as was applied (800 °C) does not allow obtaining crystallised NiAl2O4 phase.
The crystallites size in all materials studied are nanometric, as determined from the ZrO2 (011) and NiO (002) peaks broadening. The sizes of zirconia and nickel oxide crystallites are within the range of 15 ± 3 nm and 30 ± 3 nm, respectively.
In Fig. 4, the SEM images obtained for fractures of disc pellets of pure 3YSZ, 3YSZ doped by 1.0 mass% Al2O3 and the same with 50 mass% of nickel sintered in 1,200 °C are presented (except Ni/Al2O3–3YSZ sample, which was treated by hydrogen atmosphere before microscopic analysis, all the other samples were analysed directly after calcination). These SEM images confirm the X-ray analysis results—all materials are composed of nanometric particles, but NiO, ZrO2 and Al2O3 particles cannot be distinguished with SEM method. Figure 4d shows that cermet (Ni/Al2O3–3YSZ) samples have porous structure which results from nickel oxide reduction in H2 atmosphere and evolving of H2O from material. The EDS methods allowed determining chemical composition of the samples. For all samples containing Al2O3, the presence of alumina was confirmed and in Fig. 5 a model EDS image obtained for (50 %Ni/1.0 %Al2O3–3YSZ) sample is presented (respective SEM image for this sample is shown in Fig. 4d).
As mentioned above, the TEC value for anode material is an important parameter from the point of view of thermal and mechanical compatibility between electrolyte and anode materials. The TEC were calculated for all samples, using the linear regression approximation within 200–750 °C temperature range (Fig. 6). TEC values obtained for Al2O3–3YSZ samples are close to those found for fully dense 3YSZ (10.5 × 10−6 K−1) and porous 4YSZ (9.84 × 10−6 K−1) [19], but differ from TEC measured for pure nickel (17.0 × 10−6 K−1). The TEC values measured for Ni/Al2O3–3YSZ material are higher, but more close to values characteristic for 3YSZ than for nickel (Table 1) which allow one to use it as anode material for SOFC. The values of TEC obtained for samples with nickel are similar to each other.
In order to verify the influence of Al2O3 addition on electrical properties of cermets, the measurements of impedance were carried out in the temperature range of 20–750 °C and it turned out that all samples exhibit typical ionic conductivity (Fig. 7). Conductivity values measured for all alumina modified materials are higher than those for pure yttria-stabilised zirconia (Fig. 7). Moreover, the conductivity decreases with the fraction of aluminium. Since from the point of view of practical application as anode materials, the high temperature properties are the most important ones, the results of the analysis of impedance measurements carried out at 700 °C are presented here. The results of density calculations carried out for 3YSZ and Al2O3–3YSZ samples sintered at 1,200 °C are presented in Table 2. These results are in good correlation with respective values of conductivity: the highest density of sample (0.5 %Al2O3/3YSZ) corresponds with the highest value of conductivity. This results are consistent with the observations mentioned earlier about effect of alumina addition on improving of sintering temperature of zirconia materials. It is worth to noticed that higher amount of alumina in sample results in lower density and conductivity of materials.
Material containing nickel shows an increase of electrical resistivity with temperature (in the range of 20–750 °C)—a typical behaviour for metallic conductivity. In Fig. 8, the comparison of impedance spectra measured at 700 °C for 50 %Ni/Al2O3–3YSZ material sintered in 800 and in 1,200 °C with respective ones obtained for 50 %Ni/8YSZ cermet material, prepared in the same way, i.e. modified citric method are presented. In the latter case, one can see differences in the values of conductivity measured for samples sintered in 800 and 1,200 °C (the higher values in 1,200 °C are due to the increased material density and nickel concentration) while in the former these values are almost the same, regardless of sintering temperature (probably due to sintering temperature decrease caused by aluminium presence).
Conclusions
Modified citric method can be used with success for synthesis of Ni/3YSZ and Ni/Al2O3–3YSZ nanopowders. On the basis of TG/DTA and EGA curves, the thermal treatment conditions were determined and by means of thermal analysis methods the effect of oxide incorporation to non-stoichiometric oxides was confirmed.
The amount of alumina added to cermet material was too small to be detected in XRD analysis but sufficient to be visible by EDS analysis. The presence of alumina does not influence TEC values of materials. The addition of alumina to 3YSZ (on the precipitation stage) improves the ionic conductivity of material and in comparison to pure 3YSZ (the respective values are twice as high) which suggests that the presence of aluminium can indirectly improve metallic conductivity by influence on sintering temperature.
References
Jiang SP, Chan SH. A review of anode materials development in solid oxide fuel cells. J Mater Sci. 2004;39:4405–39.
Ghatee M, Shariat MH, Irvine JTS. Investigation of electrical and mechanical properties of 3YSZ/8YSZ composite electrolyte. Solid State Ionics. 2009;180(1):57–62.
Hassan AAE, Menzler NH, Blass G, Ali MA, Buchkremer HP, Stöver D. Influence of alumina dopant on the properties of yttria-stabilized zirconia for SOFC applications. J Mater Sci. 2002;37:3467–75.
Tekieli S, Demir U. Colloidal processing, sintering and static grain growth behavior of alumina-doped cubic zirconia. Ceram Int. 2005;31:973–80.
Cologna M, Contino AR, Montinaro D, Sglavo VM. Effect of Al and Ce doping on the deformation upon sintering in sequential tape cast layers for solid oxide fuel cells. J Power Sour. 2009;193:80–5.
Guo X, Siegle W, Fleig J, Maier J. Role of space charge in the grain boundary blocking effect in doped zirconia. Solid State Ionics. 2002;154–155:555.
Peters A, Korte C, Hesse D, Zakharow N, Janek J. Ionic conductivity and activation energy for oxygen ion transport in superlattices. The multilayer system CSZ (ZrO2 + CaO)/Al2O3. Solid State Ionics. 2007;178(1):67–76.
Marinsek M, Zupan K. Microstructure evaluation of sintered combustion-derived fine powder NiO–YSZ. Ceram Int. 2010;36:1075–82.
Kakade MB, Ramanathan S, Das D. Gel-combustion, characterization and processing of porous Ni–YSZ cermet for anodes of solid oxide fuel cells (SOFCs). Ceram Int. 2011;37:195–200.
Chinarro E, Figueiredo FM, Mather GC, Jurado JR, Frade JR. Combustion synthesis and characterization of Ni–MO–YSZ (M = Mg, Ca, Al2/3) cermet anodes for SOFCs. J Eur Ceram Soc. 2007;27:4233–6.
Sato K, Okamoto G, Naito M, Abe H. NiO/YSZ nanocomposite particles synthesized via co-precipitation method for electrochemically active Ni/YSZ anode. J Power Sour. 2009;193:185–8.
Banerjee S, Kumar A, Devi PS. Preparation of nanopartlicles of oxides by the citrate-nitrate process. J Therm Anal Calorim. 2011;104:859–67.
Wyrzykowski D, Hebanowska E, Nowak-Wiczk G, Makowski M, Chmurzyński L. Thermal behavior of citric acid and isometric aconitic acids. J Therm Anal Calorim. 2011;104:731–5.
Marinsek M, Zupan K, Meek J. Ni–YSZ cermet anodes prepared by citrate/nitrate combustion synthesis. J Power Sour. 2002;106:178–88.
Mularidharan P, Prakash P, Venkateswarlu M, Satyanarayana N, Sol-gel synthesis and structural characterization of nanocomposite powder: NiAl2O4:SiO2. vol 3. USA: NSTI Nanotech, Taylor & Francis; 2004. http://www.nsti.org/publications/Nanotech/2004/pdf/B3-87.pdf
KCh Stella, Nesaraj AS. Effect of fuels on the combustion synthesis of NiAl2O4 spinel particles. Iranian J Mater Sci Eng. 2010;7(2):36–44.
Pettit FS, Randklev EH, Felten EJ. Formation of NiAl2O4 by solid state reaction. J Am Ceram Soc. 1966;49(4):199–203.
CR He, Wang WG. Alumina doped Ni/YSZ anode materials for solid oxide fuel cells. Fuel Cells. 2012;9(5):630–5.
Drożdż-Cieśla E, Wyrwa J, Broś J, Rękas M. Structural, microstructural, thermal and electrical properties of Ni/YSZ cermet materials. J Therm Anal Calorim. 2012;108:1051–7.
Acknowledgements
The study was financially supported by Polish Ministry of Science and Higher Education—Grant AGH No. 11.11.160.110.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Drożdż-Cieśla, E., Wyrwa, J. & Rękas, M. Properties of Ni/YSZ cermet materials with addition of Al2O3 . J Therm Anal Calorim 113, 425–430 (2013). https://doi.org/10.1007/s10973-013-3167-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3167-3