Abstract
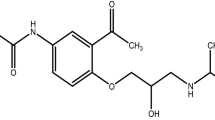
Thermal techniques, differential scanning calorimetry (DSC), and hot stage microscopy (HSM) have been used to study the interactions between furosemide and caffeine that are known to form a 1:1 cocrystal. This system has been used as an example to study the probable mechanism of cocrystal formation when the individual components, which are polymorphic, are heated. The study indicates that the phase transition of the low temperature stable polymorph of furosemide initiates cocrystal formation. This result suggests increased mass transfer rate can trigger cocrystal formation. The binary phase diagram (composition–temperature plots) of furosemide–cocrystal–caffeine system was determined from the DSC curves. The results imply that the cocrystal forms eutectic with caffeine but not with furosemide. This study has thus exemplified the use of DSC in understanding binary phase system where the two components form a cocrystal.







Similar content being viewed by others
References
Aakeröy CB, Salmon DJ. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm. 2005;7:439–48.
Schultheiss N, Newman AL. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9(6):2950–67.
Childs SL, Chyall LJ, Dunlap JT, Smolenskaya VN, Stahly BC, Stahly PG. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids, molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids. J Am Chem Soc. 2004;126:13335–42.
Cheney ML, Weyna DR, Shan N, Hanna M, Wojtas L, Zaworotko MJ. Coformer selection in pharmaceutical cocrystal development: a case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J Pharm Sci. 2011;100(6):2172–81.
Good DJ, Rodriguez-Hornedo N. Solubility advantage of pharmaceutical cocrystals. Cryst Growth Des. 2009;9(5):2252–64.
Qiao N, Li M, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: an overview. Int J Pharm. 2011;419:1–11.
Berry DJ, Seaton CC, Clegg W, Harrington RW, Coles SJ, Horton PN, Hursthouse MB, Storey R, Jones W, Frisˇcˇic′ T, Blagden N. Applying hot-stage microscopy to co-crystal screening: a study of nicotinamide with seven active pharmaceutical ingredients. Cryst Growth Des. 2008;8(5):1697–712.
Monissette SL, Almarsson O, Peterson ML, Remenar JF, Read MJ, Lemmo AV, Ellis S, Cima MJ, Gardner CR. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv Drug Deliv Rev. 2004;56(3):275–300.
Frišcˇic′ T, Trask AV, Jones W, Motherwell WDS. Screening for inclusion compounds and systematic construction of three-component solids via liquid-assisted grinding. Angew Chem Int Ed. 2006;45:7546–50.
Trask AV, Motherwell WDS, Jones W. Solvent-drop grinding: green polymorph control of cocrystallisation. Chem Commun. 2004;7:890–1.
Zhang GGZ, Henry RF, Borchardt TB, Lou XC. Efficient co-crystal screening using solution-mediated phase transformation. J Pharm Sci. 2007;96(5):990–5.
Bettinetti G, Caira MR, Callegari A, Merli M, Sorrenti M, Tadini C. Structure and solid-state chemistry of anhydrous and hydrated crystal forms of the trimethoprim-sulfamethoxypyridazine 1:1 molecular complex. J Pharm Sci. 2000;89(4):478–89.
Davis RE, Lorimer KA, Wilowski MA, Rivers JH, Wheeler KA, Bowers J. Studies of phase relationships in cocrystal systems. ACA Trans. 2004;39:41–61.
Stahly PG. Diversity in single-and multiple-component crystals: the search for and prevalence of polymorphs and co-crystals. Cryst Growth Des. 2007;7(6):1007–26.
Lu E, Rodriguez-Hornedo N, Suryanarayanan R. A rapid thermal method for cocrystal screening. CrystEngComm. 2008;10:665–8.
Chadwick K, Davey R, Cross W. How does grinding produce co-crystals? Insights from the case of benzophenone and diphenylamine. CrystEngComm. 2007;9(9):732–4.
Rothenberg G, Downie AP, Raston CL, Scott JL. Understanding solid/solid organic reactions. J Am Chem Soc. 2001;123:8701–8.
Goud NR, Gangavaram S, Suresh S, Pal S, Manjunatha SG, Nambiar S, Nangia A. Novel furosemide cocrystals and selection of high solubility drug forms. J Pharm Sci. 2012;101(2):664–80.
Jagadeesh BN, Cherukuvada S, Thakuria R, Nangia A. Conformational and synthon polymorphism in furosemide. Cryst Growth Des. 2010;10(4):1979–89.
Matsuda Y, Tatsumi E. Physicochemical characterization of furosemide modifications. Int J Pharm. 1990;60:11–26.
da Silva RC, Semaan FS, Novák C, Cavalheiro ETG. Thermal behavior of furosemide. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-2058-8.
Babilev PV, Chiripitko VV. Physicochemical and biopharmaceutic study of polymorphous caffeine modifications. Farm Zh. 1985;2:61–4.
Mazel V, Delplace C, Busignies V, Faivre V, Tchoreloff P, Yagoubi N. Polymorphic transformation of anhydrous caffeine under compression and grinding: a re-evaluation. Drug Dev Ind Pharm. 2011;37(7):832–40.
Edwards HGM, Lawson E, de Matas M, Shields L, York P. Metamorphosis of caffeine hydrate and anhydrous caffeine. J Chem Soc Perkin Trans. 1997;2:1985–90.
Pirttimäki J, Laine E, Ketolainen J, Paronen P. Effects of grinding and compression on crystal structure of anhydrous caffeine. Int J Pharm. 1993;95:93–9.
Hedoux A, Decroix A, Guinet Y, Paccaou L, Derollez P, Descamps M. Low- and high-frequency Raman investigations on caffeine: polymorphism, disorder and phase transformation. J Phys Chem B. 2011;115(19):5746–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pal, S., Roopa, B.N., Abu, K. et al. Thermal studies of furosemide–caffeine binary system that forms a cocrystal. J Therm Anal Calorim 115, 2261–2268 (2014). https://doi.org/10.1007/s10973-013-3031-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3031-5