Abstract
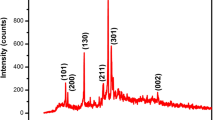
Pyrimidines and its derivatives find different pharmaceutical applications. The n-butyl 4-(3,4 dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4 tetrahydropyrimidine-5-carboxylate (abbreviated as n-butyl THPM) was synthesized. The n-butyl THPM crystals were grown by slow solvent evaporation technique using chloroform as a solvent. Yellowish, coagulated, and semi-transparent crystals having dimensions of 2 × 1.5 mm were grown. The crystals were characterized by powder XRD, FT–IR, SEM, TG–DTA–DSC, 1H-NMR, and dielectric study. The crystals remained stable up to 150 °C and then started decomposing. The DSC suggested both endothermic and exothermic reactions. One broad exothermic peak was observed at 540.3 °C due to complete decomposition of the sample into the gaseous phase and reaction within the products. 1H-NMR spectrum has been carried out to explain the molecular structure. The dielectric study was carried out in the frequency range from 50 Hz to 5 MHz at room temperature. The dielectric constant decreased as the frequency of the applied field increased. The variations of dielectric loss, a.c. conductivity, and a.c. resistivity were also studied with the frequency of the applied field.










Similar content being viewed by others
References
Kappe CO. 100 years of the biginelli dihydropyrimidine synthesis. Tetrahedron. 1993;49:6937–63.
Rovnyak CG, Atwal KS, Hedberg AJ, Kimball SD, Moreland S, Gougoutas JZ, O’Reilly BC, Schwartz J, Malley MF. Dihydropyrimidine calcium channel blockers 4. Basic-3-substituted-4-aryl 1,4-dihydropyrimidine-5-carboxylic acid esters, potent hypertensive drug. J Med Chem. 1992;35:3254–63.
Khania EL, Sillisnietse GO, YaYa O, Dabur G, Yakimenis AA. Synthesis and pharmacological investigation of some derivatives of 1,2,3,4-tetrahydropyrimidine-5-carboxylic acid. Khim Pharm Zh. 1978;78:1321–4.
Cho H, Ueda M, Shima K, Mizuno A, Hayashimatsu M, Ohnaka Y, Takeuchi Y, Hamaguchi M, Aisaka K. Dihydropyrimidines: novel calcium antagonists with potent and long lasting vasodilative and hypertensive activity. J Med Chem. 1989;32:2399–406.
Bruce MA, Pointdexter GS, Johnson G. Preparation of dihydropyrimidones as NPY antagonists. PCT Int. WO 9833791. 1998.
Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A, O’Reilly BC. Dihydropyrimidine calcium channel blockers, 3,3-carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidine carboxylic acid esters as orally effective hypertensive agents. J Med Chem. 1991;34:806–11.
Rover GJ, Dzwonczyk S, McMullen DM, Normandin DE, Parham CS, Sleph PG, Moreland SJ. Pharmacologic profile of the dihydropyrimidine calcium channel blockers SQ 32,547 and SQ 32, 926. J Cardiovasc Pharmacol. 1995;26:289–94.
Croci T, Blanchetti A, Manara L. Trifluoromethylphenyltetrahydropyrimidines for the treatment and/or prophylaxis of intestinal motility disorders. US Patent. 1992;5109005.
Malin G, Lapidot A. Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J Bacteriol. 1996;178:385–95.
Malin G, Lakobashvili R, Lapidot A. Effect of tetrahydropyrimidine derivatives on protein–nucleic acids interaction: type II restriction endonucleases as a model system. J Biol Chem. 1999;274:6920–9.
Yasuyuki G, Makoto U. Pyrimidine derivative and liquid crystal composition contain the same. US Patent. 1992;5167858.
Adrjanowicz K, Kaminski K, Paluch M, Wlodarczyk P, Grazybowska K, Wojnarowska Z, Hawelek L, Sawicki W, Lepek P, Lunio R. Dielectric relaxation studies and dissolution behavior of amorphous verapamil hydrochloride. J Pharm Sci. 2010;99:828–39.
Skoog DA, Holler FJ, Nieman TA. Principle instrumental analysis. Philadelphia: Saunders College Publishing; 1998.
Storey RA, Ym`en I. Solid state characterization of pharmaceuticals. West Sussex: Wiley; 1988.
Barker S, Antonijevic MD. Thermal analysis: dielectric techniques in solid state characterization of pharmaceuticals. West Sussex: Wiley; 1988.
Parekh BB, Purohit DH, Sagayaraj P, Joshi HS, Joshi MJ. Growth and characterization of 4-(2-hydroxyphenylamino)-pent-3-en-2-one single crystals. Cryst Res Technol. 2007;42:407–15.
Ananda Babu G, Thirupugalmani K, Ramasamy P. Growth and characterization of 4-aminopyridinium-4-nitro phenolate single crystals. Cryst Res Technol. 2009;44:675–81.
Hughes G, Wang C, Batsnov AS, Fern M, Frank S, Bryce MR, Perepichka IF, Monkman AP, Lyons BP. New pyrimidine- and fluorene-containing oligo(arylene)s: synthesis, crystal structures, optoelectronic properties and a theoretical study. Org Biomol Chem. 2003;1:3069–77.
He W, Sun HS, Xu Y, Tang S, Guo C. Methyl 4-(4-fluorophenyl)-6-isopropyl-2-(methylamino)pyrimidine-5-carboxylate. Acta Crystallogr E. 2007;63:o4157.
Gazivoda T, Raic-Malic S, Hergold-Brundic A, Cetina M. Synthesis and structural characterization of the 5-(2-haloethyl)pyrimidines-hydrogen-bonded chains in α-(1-carbamyliminomethylene)-γ-butyrolactone. Molecules. 2008;13:2786–95.
Quiroz TH, Ortega SH, García MS. Crystal structure of a quinolone antibiotic 8-ethyl-5,8-dihydro-5-oxo-2-(1-pyrrolidinyl)pyrido [2,3-d] pyrimidine -6-carboxylic acid. Anal Chem. 1999;15:105–7.
Marino MN, Salas JM. Thermal studies in metal complexes of -5-nitroso-pyrimidine derivatives. J Therm Anal Calorim. 1984;29:1053–9.
Garzón RL, Valero DG, Valenzuela C, Cruzpèrez CN, Rodriguez AG. Metal complexes of two 7-oxo-vic-triozol [4,5-d] pyrimidine derivatives. Mono Für Chemie. 1987;118:553–66.
Bruice PY. Organic chemistry. New Delhi: Pearson Education; 2002.
Enakshi D, Rao KV. Effect of high d.c. electric field on the dielectric dispersion of quenched and X-ray-irradiated NaCl and KCl single crystals. J Mater Sci Lett. 1985;4:1298–301.
Dabhi RM, Parekh BB, Joshi MJ. Dielectric studies of gel grown zinc tartrate crystals. Ind J Phys. 2005;79:503–7.
Yaghmour SJ. Ac conductivity and dielectric measurements of bulk pyronine G(Y). Eur Phys J Appl Phys. 2010;49:10402.
Acknowledgements
The authors are thankful to UGC, New Delhi, for the SAP. The authors are thankful to State Government of Gujarat for providing financial assistance to develop characterization facilities and Prof. P. H. Parsania (HOD of Chemistry Dept.) for his keen interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vyas, P.M., Pansuriya, A.M., Naliapara, Y.T. et al. Spectroscopic, thermal, and dielectric studies of n-butyl 4-(3,4-dimethoxyphenyl)-6-methyl-2-thioxo-1,2,3,4 tetrahydropyrimidine-5-carboxylate crystals. J Therm Anal Calorim 114, 839–844 (2013). https://doi.org/10.1007/s10973-013-3016-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3016-4