Abstract
The kinetics of glass transition in selenide glasses As10.2Se89.8 and As9Se90Bi in early stage of physical ageing process has been investigated by parallel differential scanning calorimetry (DSC) and exoelectron emission (EEE). It has been found that the glass transition process occurring in investigated glasses is evidenced by peaks on EEE intensity and DSC curves. Admixture of bismuth causes a distinct lowering of the temperature of glass transitions process both in the surface layer and in the volume. The addition of Bi causes a decrease in the value of the activation energy for glass transition process in both the volume and in the surface layer, thus reducing the thermal stability of investigated glasses. Physical ageing in Se-rich chalcogenide glasses leads to a significant increase of endothermic peak area A, temperature of glass transition T g and decrease of the activation energy value E. All these effects are strongly dependent on glass composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitreous chalcogenide semiconductors are known for long time as materials for potential applications, e.g. in optoelectronics [1–3]. These promising applications of chalcogenide glasses are, however, limited by the instability of their atomic structure resulting from the production process, in which the liquid phase is quenched to the ambient temperature [3]. Consequently, they change their physical properties tending to thermodynamic equilibrium at proper combination of temperature and time. This process, known as natural physical ageing [4–6], leads to the achievement of the metastable state characteristic of the supercooled liquid [1].
It was shown recently for As–Se and Ge–Se binary chalcogenide glasses that the distinguishing physical ageing process occurs in Se-rich samples [4, 5]. Physical ageing effect in chalcogenide glasses consists of two components called short-term and long-term physical ageing, respectively [4, 9]. However, the actual mechanisms and explanation of them are still lacking. Moreover, distinction between the above-mentioned components is rather arbitrary [10]. Physical ageing effect can usually be studied by differential scanning calorimetry (DSC) method [4]. Temperature dependencies of the DSC signal show an endothermic peak as a result of the structural relaxation processes at the softening point [7, 8].
The thermal stability of amorphous materials may be characterized by the activation energies and temperatures of the glass transition and crystallization processes. One of the factors limiting potential applications of chalcogenide glasses (amorphous materials) is the fact that the parameters determining the thermal stability and kinetics of physical ageing processes in the surface layer and in the volume may differ among themselves. About 20 years ago, we elaborated a method [11] enabling the determination of the parameters of thermal stability for both the surface layer and volume of amorphous materials by parallel measurements of the temperature dependencies of the EEE intensity and DSC signal. This method was already successfully applied for monitoring the phase transitions in chalcogenide glasses [12–14].
It was shown recently [15] that the modification of the structure of arsenic selenide glasses by the addition of bismuth leads to the formation of coordination defects and, with increasing concentration of Bi, to the formation of nanocrystallites in the vitreous matrix. The existence of such imperfections may lead to the lowering of potential barriers for phase transitions in the investigated arsenic selenide glasses.
The purpose of the present study is to continue studies of the effect of Bi admixture on the kinetics of the structural transitions processes in the volume and in surface layer of arsenic selenide glasses. Physical ageing effect, in its early stage, has been studied by DSC and exoelectron emission (EEE) methods.
Experimental
The investigated bulk arsenic selenide glasses As10.2Se89.8 and As9Se90Bi were prepared in the Moscow Institute of General and Inorganic Chemistry of the Russian Academy of Sciences by melting of the appropriate mixture of high purity (99,999 %) elements. Standard rejuvenation procedure (tempering at about 50 K above T g, followed by cooling to the room temperature at a rate of 5 K min−1) [4, 10] was used to obtain glassy state close to the initial the as-prepared one. After rejuvenation process, the samples were stored in the dark at normal conditions. Storage time was equal to 1 h, 1 day (1 d), 1 week (1 w) and 1 month (1 m).
Measurements of the temperature dependencies of photostimulated EEE intensity were carried out by means of the arrangement described in [16]. An open point counter with saturated ethanol quenching vapour was used for detecting the exoelectrons. The sample temperature, controlled using a Ni–CrNi thermocouple with an accuracy of about ±3 K, was changed at four constant heating rates (2, 5, 10 and 20 K min−1). The sample surface was irradiated during the measurements by unfiltered radiation from a quartz lamp using a Q-400 burner.
The calorimetric investigations of the volume glass transition were performed at five heating rates (0.5, 1, 2, 5 and 10 K min−1) using the NETZSCH DSC 404/3/F differential scanning calorimeter with E-type thermocouple. The DSC apparatus has been pre-calibrated using pure metals (In, Sn, Zn and Al) and a sapphire disc. Empty crucible made of high density Al2O3 was used as the reference. The glass transition temperature T g was determined from the DSC traces in cross-point of tangents at the beginning of the transformation (so-called “onset” T g value) using Proteus® software [20]. The same software was used for the determination of the area A of endothermic peak corresponding to the glass transition. The accuracy of the T g determination was about ±0.5 K and the relative error of the peak area determination was better than 5 %.
All the EEE and DSC measurements were performed in air atmosphere under ambient pressure.
Results and discussion
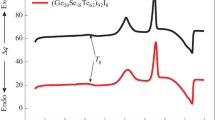
Temperature dependencies of the DSC signal and of the EEE intensity from As10.2Se89.8 and As9Se90Bi (1 m samples) are shown in Fig. 1. For all types of the investigated chalcogenide glasses, the registered temperature dependencies of DSC signal and the EEE intensity are very similar and typical of the Se-rich glasses [13, 14, 17]. All of them display single peaks corresponding to the glass transition in the surface layer (EEE) or in the volume (DSC) of samples. Admixture of bismuth causes a distinct lowering of the glass transition temperature both in the surface layer and in the volume. A comparison of the areas A under the endothermic peak (Table 1) indicates that for the (1 m)-type sample with Bi the area A is lower than that for sample without Bi. The area A is directly proportional to the enthalpy loss caused by physical ageing [4, 5].
The measurements of the temperature dependencies of the EEE intensity performed at four heating rates ranging from 2 to 20 K min−1, together with the registration of temperature dependencies of the DSC signal performed at five heating rates ranging from 0.5 to 10 K min−1 enabled to study the kinetics of the glass transition in investigated chalcogenide glasses. These measurements were used to determine the activation energies for both the volume and the surface glass transition process. Exemplary temperature dependencies of the DSC signal and photostimulated EEE intensity for As9Se90Bi (1 m) chalcogenide glass, registered at different heating rates, are shown in Fig. 2. The temperatures of the peaks observed on the EEE and DSC curves (Fig. 2) systematically shift towards higher values with increasing the heating rate. This confirms that the process responsible for the occurrence of these peaks (glass transition) is a thermally activated process.
The activation energies for the surface and volume glass transition processes observed in the EEE and DSC experiments, correspondingly, have been determined by the method proposed by Ozawa [18] by the well-known Ozawa relation:
where, V is the heating rate, A is the constant, E is the activation energy, k is the Boltzmann’s constant and T g is the glass transition temperature. The glass transition temperatures, T g, have been determined from positions of the maximum on the EEE curves [16] and as the “onset” T g value on the DSC [19, 20] curves, and the so-called Ozawa’s plots ln V = f(1/T g) were constructed. Ozawa’s plots for As9Se90Bi (1 m)-type samples are shown on Fig. 3. The slope of the Ozawa’s plot multiplied by the Boltzmann’s constant gives the value of activation energy. The value of activation energy for the surface glass transition is distinctly smaller than that for volume. In the case of the As10.2Se89.8 glass, the activation energy for volume glass transition amounts 1.89 eV and that for the surface only 0.76 eV. The accuracy of the determination of the activation energy for both the volume and surface glass transition was better than ±0.1 eV. Similar effect was observed for metallic and others chalcogenide glasses [11–14]. The same procedure, on the basis of DSC measurements, was applied for determining the activation energies of volume glass transition for all types of samples.
Figure 4 shows typical DSC traces illustrating natural physical ageing by the example of As9Se90Bi chalcogenide glass. All traces were measured in the first heating run at the same heating rate of 2 K min−1. It can be seen that physical ageing under normal conditions (in the dark) markedly increased the area of endothermic peak and the glass transition temperature. The area A under the endothermic maximum on the DSC traces is directly proportional to the enthalpy loss caused by physical ageing process [20]. With increasing storage time, the area under endothermic peak increases, which means that the glass is closer to the thermodynamic equilibrium state of the supercooled liquid [20].
Effect of storage time on the kinetics of enthalpy loss (represented by the area A under endothermic peak), temperature T g and activation energy E of glass transition process in As9Se90Bi chalcogenide glass is shown in Fig. 5. Increase of the storage time leads to considerable increase of enthalpy loss [21] and temperature of glass transition and to a decrease in the value of activation energy of this process. Short-term physical ageing is connected with the alignment of chalcogen polymeric chains of three or more chalcogen atoms followed by a shrinkage of the surrounding network [9, 20]. The process mentioned above can be activated at room temperature. Short-term physical ageing process was observed in Se-rich AsxSe100−x (x < 25) chalcogenide glasses in which the glass structure contains elements with three or more Se atoms in the chain. This explanation agrees well with the ‘chains crossing’ model validated for Se-rich As–Se glasses.
The experimentally determined values of parameters characterizing the physical ageing process in chalcogenide glasses are collected in Table 1.
The data presented in the present work indicate that
-
1.
admixture of bismuth causes a distinct lowering of the temperature of glass transitions process both in the surface layer and in the volume,
-
2.
the addition of Bi causes a decrease in the value of the activation energy for glass transition process in both the volume and in the surface layer, thus reduces the thermal stability of investigated glasses,
-
3.
physical ageing in Se-rich chalcogenide glasses leads to significant increase of endothermic peak area A, temperature T g of the volume glass transition and decrease of activation energy E values. These effects are strongly dependent on glass composition,
-
4.
the effects observed in the present study are probably connected with elementary relaxation processes within Sen (n ≥ 3) chains leading to the shrinkage of under-constrained network.
Further studies are in progress.
References
Saiter JM. Physical ageing in chalcogenide glass. J Optoelect Adv Mat. 2001;3:685–94.
Bureau B, Zhang XH, Smektala F, Adam JL, Troles J, Ma H, Boussard-Pledel C, Lucas J, Lucas P, LeCoq D, Riley MR, Simmons JH. Recent advances in chalcogenide glasses. J Non-Cryst Solids. 2004;345&346:276–83.
Ohta T, Birukawa M, Yamada N, Hirao K. Optical recording; phase-change and magneto-optical recording. J Magn Magn Mater. 2002;242&245:108–15.
Saiter JM, Arnoult M, Grenet J. Very long physical ageing in inorganic polymers exemplified by the Ge x Se1−x vitreous system. Phys B. 2005;355:370–6.
Golovchak R, Górecki Cz, Kozdraś A, Shpotyuk O. Physical ageing effects in vitreous arsenic selenides. Sol State Commun. 2006;137:67–9.
Tonchev D, Kasap SO. Effect of aging on glass transformation measurements by temperature modulated DSC. Mat Sci Eng. 2002;A328:62–6.
Elabbar AA. Glass transition in As10Se90 chalcogenide glass: a kinetic study. Phys B. 2008;403:4328–32.
Golovchak RYa, Kozyukhin SA, Kozdraś A, Shpotyuk OI, Novotortsev VM. Physical ageing of chalcogenide glasses. Inorg Mater. 2010;46:911–3.
Golovchak R, Shpotyuk O, Kozdraś A, Bureau B, Vlcek M, Ganjoo A, Jain H. Atomistic model of physical ageing in Se-rich As–Se glasses. Phil Mag. 2007;87:4323–34.
Golovchak R, Kozdraś A, Shpotyuk O, Kozyukhin S, Saiter JM. Long-term ageing behaviour in Ge–Se glasses. J Mater Sci. 2009;44:3962–7.
Górecki Cz, Górecki T, Michno Z. Thermal stability of the Fe40-xNi40CrxB20 metallic glasses as studied by the EEE and DTA methods. Acta Phys Polon. 1987;A72:157-9. Thermal stability of the Ni78SixB22-x metallic glasses as studied by the EEE and DTA methods. Acta Phys Polon. 1987;A72:161-3.
Górecki Cz, Górecki T, Golovchak R, Żurawska A, Wacke S. Kinetics of phase transitions in semiconducting amorphous chalcogenides AsxSe100-x as studied by differential thermal analysis and exoelectron emission techniques. Visnyk Lviv Univ. 2005;38:399–403.
Górecki Cz, Górecki T, Kozdraś A, Golovchak R. Kinetics of phase transitions in vitreous chalcogenide semiconductors AsxSe100-x as studied by differential thermal analysis (DTA) and exoelectron emission (EEE) methods. Visnyk Lviv Univ. 2007;40:254–60.
Górecki Cz, Górecki T, Kostrzewa M. The kinetics of structural transformations in vitreous chalcogenide semiconductors AsxSe100-x as studied by differential thermal analysis and exoelectron emission methods. Opole: Studia i Monografie nr 238. Optimization of production processes. Oficyna Wydawnicza PO. 2008;23-31.
Golovchak R, Shpotyuk O, Kovalskiy A, Miller AC, Cech J, Jain H. Coordination defects in bismuth-modified arsenic selenide glasses; High-resolution x-ray photoelectron spectroscopy measurements. Phys Rev B. 2008;77:1722011–4.
Górecki T, Sujak B. Photostimulated exoelectron emission during phase transitions of binary and ternary nickel alloys. Acta Phys Polon. 1972;A42:487–501.
Górecki Cz, Górecki T. Kinetics of phase transitions in vitreous chalcogenide semiconductors AsxSe100-x-yBiy as studied by differential thermal analysis and exoelectron emission methods. IOP Publishing. J Phys Conf Ser. 2011;289:012021.
Ozawa T. Kinetics of non-isothermal crystallization of amorphous materials. J Therm Anal. 1970;2:301–12.
Schultze D. Termiczna analiza różnicowa (Differentialthermoanalyse). Warszawa: PWN; 1974.
Golovchak R, Shpotyuk O, Kozdraś A, Riley BJ, Sundaram SK, McCloy JS. Radiation effects in physical aging of binary As–S and As–Se glasses. J Therm Anal Calorim. 2011;103:213–8.
Golovchak RYa, Kozdraś A, Shpotyuk OI. Physical ageing in glassy As–Se induced by above-bandgap photoexposure. Solid State Commun. 2008;145:423–6.
Acknowledgements
The authors are very grateful to Prof. S Kozyukhin for the kind donation of the samples of investigated materials.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Górecki, C., Górecki, T. The kinetics of phase transitions in vitreous chalcogenide semiconductors As10.2Se89.8 and As9Se90Bi in early stage of physical ageing process. J Therm Anal Calorim 114, 725–729 (2013). https://doi.org/10.1007/s10973-013-2978-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-2978-6