Abstract
The partial system ErPO4–NaPO3–Er(PO3)3 of the Er2O3–Na2O–P2O5 oxide system has been investigated by thermoanalytical methods and X-ray powder diffraction. On the basis of the obtained results the phase diagram of the partial system is proposed. The system is bounded by three subsystems: (i) ErPO4–Er(PO3)3, (ii) Er(PO3)3–NaPO3 and (iii) ErPO4–NaPO3. Their phase diagrams are proposed. In the Er(PO3)3–NaPO3 subsystem an intermediate compound NaEr(PO3)4 occurs; it melts incongruently at 655 °C. It was found that ErPO4 and NaEr(PO3)4 form a section which is a real system only in the subsolidus region (below 646 °C). Two ternary invariant points (one ternary peritectic and one ternary eutectic) occur in the investigated partial system ErPO4–NaPO3–Er(PO3)3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to literature data, both the phase-pure phosphates of rare earth elements or yttrium and the ones doped with different elements can be applied in practice, for instance as laser or phosphor materials etc. During the course of searching for new compounds and learning about their properties, an important role is played by investigations of phase equilibria which occur in binary and multicomponent systems. Accordingly, experimental researches on phase diagrams of oxide systems such as Ln2O3–M (I)2 O–P2O5, (where Ln denotes La, Ce, Nd, Er and Y; M(I) does Na, K, Rb) have been conducted in our laboratory for several years [1–9]. In this paper, the results of a study of phase equilibria in the Er2O3–Na2O–P2O5 oxide system, within the composition range ErPO4–NaPO3–Er(PO3)3 are presented.
Previous phase investigations carried out in the Er2O3–Na2O,{K2O}–P2O5 systems were rather fragmentary. There exist literature data concerning a subsystem of KPO3–Er(PO3)3 [10] and the system Ln2O3–Na2O–P2O5–H2O (where Ln denotes, among others, Er) [11]. The investigation reported in Ref. [11] was done at relatively low temperatures (350 and 500 °C). Some literature information concerns, too, starting materials such as ErPO4, Er(PO3)3 and NaPO3. The ErPO4 orthophosphate was obtained and investigated some years ago; its X-ray diffraction (XRD) data were reported in, among others, papers [12–15]. Those authors have established that ErPO4 crystallizes in the tetragonal system, space group I41/amd. Lattice constants are a = 6.8614(5) and c = 6.0082(9) Å [12]. It has been found in a thermal stability investigation [16] that ErPO4 melts congruently at about 1896 °C and occurs in one polymorphic form.
Moreover, methods of obtaining the Er(PO3)3 metaphosphate and XRD data on the compound are widely depicted in the literature, for example, in [10, 17, 18]. According to information of [10], Er(PO3)3 phosphate melts congruently at 1424 °C, and does not exhibit polymorphic transitions. Certain discrepancies which are present in the literature, however, concern the compound’s structure. It has been established that Er(PO3)3 crystallizes in the monoclinic system (space group P21/c, lattice parameters a = 11.306, b = 20.1, c = 10.076 Å, β = 97.03° [17]; space group Pm, lattice parameters a = 10.9430(30), b = 6.9710(20), c = 9.6700(20) Å, γ = 91.82(2)° [18]).
NaPO3 metaphosphate melts congruently at 627 °C and occurs in three polymorphic modifications. Transition points are α/β—at 510 °C and β/γ—at 404 °C [19]. Thermal stability of the metaphosphate has been discussed in [20]. Its different structures: monoclinic, tetragonal and orthorhombic are known.
A double metaphosphate NaEr(PO3)4 is also known. It crystallizes in the monoclinic system (space group P21/n, lattice parameters a = 7.155(15), b = 12.9870(70), c = 9.662(32) Å, β = 89.32(2)° [21]). NaEr(PO3)4, according to its stoichiometric composition, should occur as an intermediate compound in the subsystem Er(PO3)3–NaPO3, with the molar ratio of the starting metaphosphates equal to 1:1.
Experimental
Synthesis
For the investigation of the phase equilibria in the partial system ErPO4–NaPO3–Er(PO3)3, the following commercial reagents were used: Er2O3 (99.9 %), NaH2PO4·H2O, Na2CO3, (NH4)2HPO4—all analytically pure. From these reagents the phosphates NaPO3, Er(PO3)3, NaEr(PO3)4 and ErPO4 were synthesized.
Er(PO3)3 was obtained from (NH4)2HPO4 and Er2O3. The compounds were weighed in stoichiometric amounts according to the reaction: 6(NH4)2HPO4 + Er2O3 → 2Er(PO3)3 + 12NH3 + 9H2O. Then the compounds were thoroughly mixed, rubbed in agate mortar and then sintered at 450 °C for 24 h and at 700 °C for 24 h.
ErPO4 was synthesized from the same compounds and in a similar way as Er(PO3)3, but in this case the reaction was: 2(NH4)2HPO4 + Er2O3 → 2ErPO4 + 4NH3 + 3H2O. The compounds were sintered at 450 °C for 24 h, at 900 °C for 24 h and finally at 1050 °C for 2 days.
Erbium and sodium metaphosphate NaEr(PO3)4 was synthesized via the solid-state reaction from Na2CO3, (NH4)2HPO4 and Er2O3. The substrates were weighed in stoichiometric amounts according to the reaction: Na2CO3 + 8(NH4)2HPO4 + Er2O3 → 2NaEr(PO3)4 + CO2 + 16NH3 + 12H2O. Mixing and grounding procedures were as above. The compounds were sintered at 200 °C for 24 h and at 500 °C for 10 days.
Sodium metaphosphate NaPO3 was obtained from NaH2PO4·H2O by dehydration at 300 °C for 1 h and at 500 °C for 1 h [22].
Samples for thermoanalytical and XRD investigations were presynthesized by solid-state reaction. The substrates were weighed out in assumed amounts, thoroughly mixed and ground in agate mortar and then sintered. The conditions for the presynthesis (i.e. the sintering temperature and time) were found experimentally.
Measurement equipment
The thermoanalytical investigations were carried out using a SETSYS™ (TG-DSC 1500; SETARAM) differential thermal analyser (calorimeter) with a balance. The samples (weighing from 0.010 to 0.018 g) were placed in platinum crucibles and heated in a temperature range of 20–1300 °C, at a rate of 10 °C min−1 under argon. The DTA/TG experiments were carried out using a derivatograph type 3427 (MOM, Hungary). The analytical conditions were: a temperature range of 20–1400 °C, a heating rate of 5 °C min−1, platinum crucibles under air, a sample weight of 0.4–0.6 g. Temperature was measured by means of a Pt/PtRh10 thermocouple which was calibrated against the melting points of NaCl, K2SO4, Ca2P2O7 and the transition point of K2SO4.
A thermal ‘static’ method and the quenching-in-air technique were also applied to determine the melting temperature of the samples. In the thermal ‘static’ method a sample is heated at a constant temperature and next it is quenched; more details are described in section "Results and discussion". A high-temperature vertical-tubular furnace (a temperature range of 20–1750 °C; Nabertherm RHTV 120-300/18) was used in these investigations. The temperature read-out accuracy was ±5 °C.
The phase purity of the parent phosphates and the phase composition of the samples of the investigated system were controlled by XRD at room temperature. A SIEMENS D 5000 diffractometer with CuKα radiation, Ni filter, was used.
Results and discussion
The phase equilibria were investigated by the thermoanalytical methods, XRD and ‘static’ thermal techniques. The phase equilibria that occur in the partial system ErPO4–NaPO3–Er(PO3)3 has not previously been reported. This area is surrounded by three subsystems: (i) ErPO4–Er(PO3)3, (ii) Er(PO3)3–NaPO3 and (iii) ErPO4–NaPO3. Their phase diagrams were also earlier unknown. The first two subsystems have a common starting component of Er(PO3)3. According to [10], the compound melts congruently at 1424 °C, though at a temperature of about 1290 °C its decomposition starts according to the reaction:
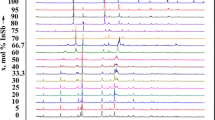
In view of the above, investigation was started with verifying the information. Based on the DTA and TG curves of the Er(PO3)3 metaphosphate, it was determined that a small mass loss in the solid phase slowly appeared at a temperature of about 1200 °C (see Fig. 1). For samples quenched from high temperatures of 1250, 1300, 1350 and 1400 °C, XRD patterns revealed reflections typical of ErPO4. Moreover, the reflection intensity increased with increasing temperature of quenching the Er(PO3)3 sample, and the reflections from the Er(PO3)3 sample gradually declined. Accordingly, in order to find the congruently melting point of the Er(PO3)3 metaphosphate, a ‘static’ thermal method was employed. Thus, several portions of the Er(PO3)3 metaphosphate were prepared and pelleted. Next each pellet (in a platinum crucible) was placed into the furnace earlier warmed up to assumed temperatures of the range from 1400 to 1480 °C. After a 5-min period, the crucible containing a pellet was quickly cooled down in the air. The short heating time was used for minimizing the decomposition of the Er(PO3)3 metaphosphate. In such a way, it was found that Er(PO3)3 melts congruently at a temperature of about 1480 °C (at lower temperatures the pallets only sintered).
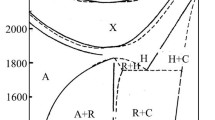
To the determine the phase diagram of the ErPO4–Er(PO3)3 subsystem, samples of unequimolar mixtures of parent phosphates (after the mechanical processing; see "Experimental") were presynthesized by the solid-state reaction. The phase composition of all sinters was checked by XRD. It was established that the sinters were a mixture of ErPO4 and Er(PO3)3. The ‘static’ method was employed to determine the phase equilibria occurring in the ErPO4–Er(PO3)3 subsystem at high temperatures. The static-method examination was carried out in the temperature range from 1300 to 1750 °C. It was found that ErPO4 i Er(PO3)3 gave a simple eutectic system. The determined eutectic transformation point was at about 1460 °C, with parameters of ca. 90 mass% Er(PO3)3 and ca. 10 mass% ErPO4. This technique applied to phase-equilibrium investigation allowed for construction of a phase diagram confined to the composition range 80–100 mass % Er(PO3)3. The phase diagram of the ErPO4–Er(PO3)3 subsystem is shown in Fig. 2. Liquidus and solidus curves are drawn with dashed line; consequently, they have a suggested shape.
Next, phase equilibria of Er(PO3)3–NaPO3 subsystem were investigated in the entire composition range at temperatures to about 1000 °C. Experimental work was started with the search for NaEr(PO3)4 double phosphate which should occur as an intermediate compound in the subsystem under discussion. The empirical formula of the metaphosphate indicates a molar ratio of 1:1 for Er(PO3)3 and NaPO3 (i.e. 20.16 mass% NaPO3 and 79.84 mass% Er(PO3)3). Accordingly, an equimolar mixture of the above metaphosphates was heated in the solid phase at different temperatures for different time intervals. The phase composition of the all obtained sinters was identified by XRD analysis. In such a way it was found that the phase-pure NaEr(PO3)4 could be obtained by a solid-state reaction of heating a mixture of 1 mol Er(PO3)3 and 1 mol NaPO3 at a temperature of 500 °C for 10 days; the reaction proceeded slowly. Thus the starting supposition was confirmed; NaEr(PO3)4 had been observed as an intermediate compound in a Er(PO3)3–NaPO3 subsystem. After employing the DTA/TG and DSC/TG techniques, it was established that NaEr(PO3)4 melts incongruently at 655 °C to give Er(PO3)3 (solid) and an NaPO3-rich liquid. DTA curve of heating (from room temperature to 655 °C) showed the occurrence of a single endothermic effect at 655 °C, which indicated the presence of a one polymorphic modification.
In order to determine the Er(PO3)3–NaPO3 phase diagram, the following samples were investigated by the thermoanalytical and XRD methods: (1) unequimolar mixtures of Er(PO3)3 and NaEr(PO3)4 metaphosphates, to determine the phase equilibria within the composition range 0–20.16 mass% NaPO3 and (2) unequimolar mixtures of NaEr(PO3)4 and NaPO3 metaphosphates, to determine the phase equilibria within the composition range 20.16–100 mass% NaPO3. The mixtures were synthesized via solid-state reaction by heating:
-
Samples NaPO3-rich (the ones that contained more than 20 mass% NaPO3) at 550 °C for 24 h;
-
Samples Er(PO3)3-rich (the ones with less than 20 mass% NaPO3) at 550 °C for 70 h.
After the mechanical processing, the obtained sinters were subjected to thermoanalytical investigations. Phase composition of all sinters was identified by XRD. Depending on the initial composition of a sample, DTA and DSC curves of heating showed one, two or three endothermic effects. An endothermic peak, related to a temperature of 655 °C, occurred within the composition range 0–54 mass% NaPO3. The effect was ascribed to a peritectic reaction:
All samples of compositions from the range 30–90 mass% NaPO3 exhibited a high endothermic peak on DTA curves, with the corresponding temperature 605 °C. It was attributed to a eutectic phase transformation. The liquidus temperature effects were observed on DTA curves only within the composition range 54–90 mass% NaPO3. For the part NaPO3-poor, any heating effects were difficult to unequivocally identify (see Fig. 3). There was, however, an exception for a sample of the 45 mass% NaPO3 content where a heating effect appeared on the DTA curve at about 855 °C. For this reason, liquidus curve of the above composition range has been drawn with dashed line as a suggested one. The peritectic reaction ended at about 54 mass% NaPO3. The eutectic present in the Er(PO3)3–NaPO3 subsystem melted at 605 °C and had a composition of ca. 80 mass% NaPO3 and ca. 20 mass% Er(PO3)3. The metaphosphate NaPO3 occurred in three polymorphic modifications. In the subsystem under discussion, however, no heating effects related to such transformations were observed on the DTA curves. The phase diagram of the Er(PO3)3–NaPO3 subsystem, elaborated on the basis of the results presented above, is shown in Fig. 4.
In the next stage of experimental work, phase equilibria occurring in the ErPO4–NaPO3 subsystem were investigated. The key question was to see whether or not the intermediate compound of the formula NaErP2O7 would occur in that system. There were, in fact, known diphosphates from lanthanides and alkali metal elements of the formula MILnIIIP2O7 [23–27]. Accordingly, an attempt was made to obtain NaErP2O7 by the conventional method of solid-state reaction using ErPO4 and NaPO3 as starting materials. Consequently, an equimolar mixture of both compounds was heated (in the solid phase) at different temperatures and using different time intervals. The phase composition of each sinter was identified by X-ray powder diffraction. XRD patterns, however, revealed only reflections typical of ErPO4 and NaPO3. Consequently, another attempt was done to obtain the NaErP2O7 diphosphate using Er2O3, Na2CO3 and (NH4)2HPO4 as the starting materials. Those compounds, mixed in the molar ratio 1:1:4, were sintered at different temperatures and time intervals. The sinters’ phase composition also was identified by XRD. This method, too, failed to give the binary phosphate of sodium and erbium. In order to determine phase equilibria and elaborate the phase diagram of the ErPO4–NaPO3 subsystem, the samples of unequimolar mixtures of starting phosphates were used. The mixtures were presynthesized (via solid-state reaction) by heating at 550 °C for 70 h. The obtained sinters were investigated by the DTA and XRD methods. Subject to a sample’s composition, one or two endothermic effect occurred on the DTA curves of heating. A rather strong effect, which appeared for all samples, was connected with a temperature of 623 °C. This effect was attributed to a eutectic phase transition. The composition at the eutectic point was ca. 86 mass% NaPO3 and ca. 14 mass% ErPO4. It should be stressed that, for few samples, it was possible to DTA identify the heating effects associated with the melting process. Due to this fact, a true and exact shape of the liquidus curve could be established only in the NaPO3-rich area, i.e. above 60 mass% NaPO3. Similar to the case of Er(PO3)3–NaPO3 subsystem, no heating effects were visible on the DTA curves of samples from the ErPO4–NaPO3 that could be ascribed to polymorphic transformations of sodium metaphosphate NaPO3. Based on the above results, a phase diagram of the ErPO4–NaPO3 subsystem has been elaborated and shown in Fig. 5.
Exact determination of phase equilibria in the ErPO4–NaPO3–Er(PO3)3 partial system, within the entire composition and temperature range, appeared to be rather complicated. Both NaEr(PO3)4 and Er(PO3)3 are high temperature unstable. Samples for the investigation were prepared from starting materials of ErPO4, Er(PO3)3, NaPO3 and NaEr(PO3)4. Unequimolar mixtures of those phosphates typically were subjected to heating at different temperatures and time intervals. The existence of the section ErPO4–NaEr(PO3)4 as found by thermoanalytical investigations and XRD allowed for elaboration of the relevant phase diagram both for that system and for the ErPO4–NaPO3–Er(PO3)3 partial system (shown in Figs. 6 and 7, respectively). Samples for investigation of the ErPO4–NaEr(PO3)4 section were obtained from the starting phosphates. Their unequimolar mixtures were initially synthesized as previously in the solid phase, by heating at 550 °C for 70 h, which was followed by cooling down to room temperature. Those sinters exhibited two successive endothermic effects of heating on the DTA curves in the entire range of composition, at corresponding temperatures of 646 and 660 °C, respectively. In contrast, a variety of endothermic effects appeared on the DTA-heating curves at higher temperatures. Those ones were weak and diffuse, however, which made an unequivocal interpretation, and thereby drawing a liquidus line, impossible. In turn, for the samples that were quenched from temperatures above 660 °C, XRD patterns revealed reflections typical of Er(PO3)3, ErPO4 and also NaPO3 (in trace amounts). Based on those results as well as theoretical consideration, it was assumed that the ErPO4–NaEr(PO3)4 section was a true quasi-binary one only in the subsolidus area, i.e. below the temperature 646 °C. Though, at higher temperatures, it would have a multiphase character. Above the 646 °C point, in fact, four phases would exist—namely a liquid L, ErPO4, Er(PO3)3 and NaEr(PO3)4. Consequently, Fig. 6 represents only data obtained from thermal analysis of heating of sinters of the ErPO4–NaEr(PO3)4 section. The suggested theoretical phase diagram of the section is shown in the upper-left corner of Fig. 6.
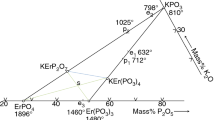
Phase diagrams of the ErPO4–NaPO3–Er(PO3)3 partial system is shown in Fig. 7. A double metaphosphate NaEr(PO3)4 is formed as a result of the peritectic reaction that goes in the side system at a constant temperature, according to the reaction:
Reaction (3), in the partial system, is manifested in the form of a quasi-ternary peritectic reaction. Indeed, a quasi-ternary peritectic reaction occurs in the field of ErPO4–P1–NaEr(PO3)4–Er(PO3)3 (the triple peritectic quadrangle), which proceeds according to the reaction:
(where L(P1) denotes a liquid of the composition corresponding to P1 point). The ternary peritectic point (according to Fig. 6) is 646 °C.
Four compounds of ErPO4, Er(PO3)3, NaPO3 and NaEr(PO3)4 are present in the system under discussion. Each of them crystallizes from the liquid phase. Fields of primary crystallization are given in Table 1. The fields are separated by eutectic and peritectic lines. A binary peritectic curve extends from point p1 to P1, along which the NaEr(PO3)4 phosphate separates according to the reaction:
From point e1 to P1, in turn, a eutectic curve extends along which the ErPO4 and Er(PO3)3 compounds separately. Both the above-mentioned curves converge at the point P1, where the quasi-ternary peritectic reaction (4) proceeds. This reaction completes solidification of alloys existing in the ErPO4–NaEr(PO3)4–Er(PO3)3 field. For the alloys from the ErPO4–P1–NaEr(PO3)4 field, an excess liquid of L(P1) arises due to the above mentioned peritectic reaction (4). Accordingly, solidification does not end at 646 °C. The solidification process occurs at a labile temperature, from 646 to 586 °C, along the eutectic curve P1E1. The ErPO4 and NaEr(PO3)4 compounds crystallize along the above eutectic curve (point P1 to E1). NaPO3 and NaEr(PO3)4 crystallize along the e2E1 eutectic curve whereas NaPO3 and ErPO4 crystallize along the e3E1 eutectic curve. Three eutectic lines, mentioned above, meet at the ternary eutectic point E1 where three phosphates NaPO3, ErPO4 and NaEr(PO3)4 crystallize at a constant temperature of 586 °C. Crystallization of alloys from the ErPO4–NaPO3–Er(PO3)3 partial system ends just at this point. Eutectic and peritectic lines in Fig. 7 are drawn dashed as their position and shape is approximate only.
References
Szuszkiewicz W. Phase equilibria in the system YPO4–NaPO3–Y(PO3)3. Pol J Chem. 1993;67:329–34.
Znamierowska T, Bandrowski S. Study of phase equilibria in the system Nd2O3–Na2O-P2O5. The partial system NdPO4–NaPO3–Nd(PO3)3. Pol J Chem. 2004;78:771–8.
Szczygieł I, Znamierowska T. Phase equilibria in the partial system CePO4-NaPO3-Ce(PO3)3. J Therm Anal. 1995;44:955–60.
Jungowska W, Znamierowska T. The system LaPO4–KPO3–La(PO3)3. Mater Chem Phys. 1991;27:109–16.
Czupińska G, Znamierowska T. The system KPO3–Y(PO3)3. J Therm Anal. 1990;36(2):639–43.
Szuszkiewicz W, Znamierowska T. Phase equilibria in the Y2O3–P2O5. Pol J Chem. 1989;63:381–91.
Szczygieł I, Znamierowska T, Mizer D. Phase equilibria in the Oxide System Nd2O3–K2O–P2O5. Solid State Sci. 2010;12:1205–10.
Szczygieł I, Matraszek A, Znamierowska T. Phase equilibria in the Ce2O3–K2O–P2O5 System. J Therm Anal Calorim. 2008;93:671–6.
Szuszkiewicz W, Radomińska E, Znamierowska T, Wilk P. Phase equilibria in the YPO4–Rb3PO4 system. J Therm Anal Calorim. 2012; doi:10.1007/s10973-011-2172-7.
Ferid M, Kbir-Ariguib N, Trabelsi-Ayedi M. Etude du diagramme d’equilibre du systeme KPO3-Er(PO3)3 et des formes allotropiques des phosphates condenses KEr(PO3)4. Thermochim Acta. 1988;136:139–47.
Sazenkhov AY, Masloboev VA, Sklokina NF, Tkachenko VG, Chaikin VP. Phase formation in the Na2O–Ln2O3–P2O5-H2O (Ln–Nd, Gd, Er) system at 350°C and 500°C. Russ J Inorg Chem (Engl Trans). 1991;36:1642–5.
Ushakov SV, Helean KB, Navrotsky A, Boatner LA. Thermochemistry of rare-earth ortophosphates. J Mater Res. 2001;16:2623–33.
Patscheke E, Fuess H, Will G. Neutron diffraction study of ErPO4 and ErVO4. Chem Phys Lett. 1968;2:47–50.
Schwarz H. Die phosphate, arsenate und vanadate der seltenen erden. Z Anorg Allg Chem. 1963;323:44–56.
Ni Y-X, Hughes JM, Mariano AN. Crystal chemistry of the monazite and xenotime structures. Am Mineral. 1995;80:21–6.
Hikichi Y, Nomura T. Melting temperatures of monazite and xenotime. J Am Ceram Soc. 1987;70(10):252–3.
Tarasenkova OS, Dorokhova GI, Chudinova NN, Litvin BN, Vinogradova NV. Phase formation in the K2O-Er2O3–P2O5–H2O System. Inorg Mater (Engl Trans). 1985;21:383–8.
Dorokhova GI, Karpov OG. The crystal structure of a new modification of TrP3O9 (Tr=Er). Sov Phys Crystallogr. 1984;29:400–2.
Berak J, Znamierowska T. Phase equlibria in the system CaO–Na2O–P2O5. Part II. The partial system Ca(PO3)2–Na2O–P2O5. Roczniki Chemii Ann Soc Chim Polonorum. 1972;46:1697–708.
Szuszkiewicz W. Phase equilibria in the system YPO4-NaPO3. Thermochim Acta. 1992;198:97–102.
Maksimova SI, Masloboev VA, Palkina KK, Sazhenkov AY, Chibiskova NT. Preparation and structure of the double polyphosphate NaEr(PO3)4. Russ J Inorg Chem (Engl Trans). 1988;33:1434–5.
Thilo E. Condensed phosphates and arsenates. In: Emeleus HJ, Sharpe AG, editors. Advances in inorganic chemistry and radiochemistry. New York: Academic Press; 1962. p. 1–59.
Anisimova NY, Trunov VK, Chudinova NN. Double diphosphates NaLnP2O7. Inorg Mater (Engl Trans). 1988;24(2):201–4.
Anisimova NY, Chudinova NN, Trunov VK. Synthesis of binary diphosphates of alkali and rare-earth metals MLnP2O7. Inorg Mater (Engl Trans). 1993;29(1):122–4.
Anisimova NY, Chudinova NN, Trunov VK. X-ray diffraction characteristics and thermal transformations in the binary phosphates MLnP2O7 (M=K, Rb, Cs; Ln=R.E.E. of yttrium subgroup). Inorg Mater (Engl Trans). 1993;29(1):125–9.
Gabelica-Robert M, Tarte P. New pyrophosphates MIMIIIP2O7. J Solid State Chem. 1983;3:475–8.
Akrim A, Zambon D, Metin J, Cousseins JC. Rietveld refinement of the RbYP2O7 structure and crystal chemistry of related rare earth diphosphates. Eur J Solid State Inorg Chem. 1993;30:483–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bandrowski, S., Znamierowska, T. Investigation of phase equilibria in the Er2O3–Na2O–P2O5 system: the partial system ErPO4–NaPO3–Er(PO3)3 . J Therm Anal Calorim 113, 105–111 (2013). https://doi.org/10.1007/s10973-012-2910-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2910-5