Abstract
The phase equilibria occurring in the ErPO4–K3PO4 system were investigated by the thermal analysis, FTIR, and X-ray powder diffraction methods. On the basis of obtained results, the related phase diagram is proposed. This system includes one intermediate compound, K3Er(PO4)2; the double phosphate melts incongruently at 1355 °C and occurs in two polymorphic forms; transformation β/α-K3Er(PO4)2 proceeds at 420 °C. The eutectic occurs at the composition of 58.5 wt% K3PO4, 41.5 wt% ErPO4 at 1317 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many papers about double phosphates of the general formula M I3 Ln(PO4)2 (where MI denotes an alkali metal and Ln is a rare earth element or Y or Sc) have been published. The information is mainly focused on the preparation methods, crystalline structure, and application possibilities of those compounds. According to the data, lanthanide-alkali metal double phosphates are of technological importance for applications in optics and electronics [1–10].
In view of relevant information from the literature, double phosphates of the formula M I3 Ln(PO4)2 should occur in the systems of LnPO4–M I3 PO4 (where Ln denotes a rare earth element or yttrium, and MI does an alkali metal). According to our research group results, such compounds occur in the Ln2O3–M I2 O–P2O5 oxide systems on the LnPO4–M I3 PO4 subsystems, where Ln = La, Ce, Nd, Y and MI = Na, K, Rb [11–18]. It should be noted that, in the system YPO4–Na3PO4, two intermediate compounds of Na3Y(PO4)2 and Na3Y2(PO4)3 occur; both compounds melt congruently. Also, in the system YPO4–Rb3PO4, two intermediate compounds occur; namely Rb3Y(PO4)2 which melts congruently, and the Rb3Y2(PO4)3 which is unstable and decomposes in the temperature range between 1300 and 1330 °C. In each of the other investigated systems, a single intermediate of M I3 Ln(PO4)2 occurs; it melts incongruently. Double phosphates M I3 Ln(PO4)2 are usually obtained in a solid-state reactions by sintering an equimolar mixture of M I3 PO4 and LnPO4.
In the present paper, the results of investigation of the ErPO4–K3PO4 subsystem are presented. The related phase diagram has not been reported so far. It is known from the literature that K3Er(PO4)2 exists as well. According to Refs. [19, 20], the compound appears in two polymorphic modifications. The high-temperature one crystallizes in the hexagonal system (S.G. \( P\mathop 3\limits^{ - } \), glaserite-type) and the low-temperature one does in the monoclinic system (S.G. P21/m, a = 7.371(1), b = 5.595(1), c = 9.318(1) Å, and β = 90.90(1)°). A polymorphic transition in K3Er(PO4)2 exhibits at 436.4 °C [19].
The parent orthophosphates ErPO4 and K3PO4 are known for congruent melting at 1896 ± 20 °C [21–23] and 1620 ± 20 °C [24], respectively. Polymorphism of both orthophosphates was investigated by many authors (see, e.g., [21, 24–31]). Erbium orthophosphate, ErPO4, is related to REPO4 group with xenotime structure, isostructural to zircon (ZrSiO4). Orthophosphate ErPO4 crystallizes in the tetragonal system (S.G. I41/amd, a = 6.8614(5), c = 6.0082(9) Å, Z = 4) [21]. The compound exists in one polymorphic form. According to the literature on K3PO4, polymorphism has revealed significant disagreements. This problem will be described in the “Results” section.
Experimental
The following commercial materials: Er2O3 (Aldrich), and NH4H2PO4, (NH4)2HPO4, K2CO3, K3PO4·3H2O (POCh)—all analytically pure were used to prepare the test samples from the ErPO4–K3PO4 system. The erbium orthophosphate ErPO4 was synthesized from Er2O3 and NH4H2PO4 by the method described in [32]. Potassium orthophosphate K3PO4 was obtained from K3PO4·3H2O by dehydration at 900 °C for 1 h.
Phase equilibria in the ErPO4–K3PO4 system were investigated by thermoanalytical methods and X-ray powder diffraction at room temperature.
Samples for investigations were presynthesized by the reaction in the solid phase. The substrates were weighed out in fixed amounts, thoroughly mixed (in weighing bottle), rubbed in an agate mortar, and then sintered. The sintering temperature and time were determined experimentally.
The DSC/TG analysis during heating was carried out using a calorimeter SETSYS™ (TG–DSC 1500; SETARAM) up to 1300 °C (heating rate: 10 K min−1, argon atmosphere, platinum crucibles; mass of samples 15–30 mg). The DTA/TG-heating was performed by means of a derivatograph type 3427 (MOM, Hungary) within temperature range of 20–1400 °C with a heating rate 5 °C min−1. Platinum crucibles and an air atmosphere were used; mass of samples was 400–600 mg. The standard substance was Al2O3. The temperatures were measured by a Pt/PtRh10 thermocouple standardized for the melting points of NaCl (801 °C), K2SO4 (1070 °C), Ca2P2O7 (1353 °C), and the transition points of K2SO4 (583 °C). The high-temperature experiments (above 1400 °C) were conducted in a horizontal resistance furnace with molybdenum winding under argon. Presynthesized samples of about 3 g mass were pressed into pellets and placed in boats made of PtRh30 alloy. Temperature points at which the samples began to melt and disappeared from the visual field were read with an optical pyrometer. For the samples that melt in a range of temperature, the points determined are approximate. The optical pyrometer was calibrated against the melting points of Na3PO4 (1583 °C) and Ca3(PO4)2 (1810 °C). In the thermal analysis, the temperature read-out accuracy was ±1.5 °C in a temperature range up to 800 and ±3 °C above 800 and ±30 °C when the temperature was read by the optical pyrometer.
The phase purity of the reagents and phase composition of the sinters and the melted samples for the investigated system were controlled by X-ray powder diffraction at room temperature. A SIEMENS D 5000 diffractometer with CuKα radiation (λ = 0.154 nm) were used. The measurements were performed in 2θ angle range of 10°–60°. The 2θ scanning speed was 0.02° s−1. A Fourier transform infrared spectra was recorded on a PERKIN-ELMER SYSTEM 2000 FTIR spectrophotometer in the range of wavenumbers 400–1300 cm−1 using KBr pressed pellets.
Results
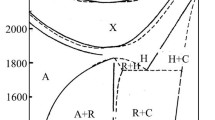
The phase diagram of the ErPO4–K3PO4 system, which was not known before, is shown in Fig. 1; it was elaborated in the entire composition range and temperatures ranging from room temperature to 1800 °C.
The experimental work was started with determining the most favorable conditions under which synthesis of the K3Er(PO4)2 double phosphate would proceed to result in giving a phase-pure compound. The following were the starting materials: (1) Er2O3, K2CO3, and (NH4)2HPO4 (mixed in the molar ratio of 1:3:4); (2) ErPO4, K2CO3, and (NH4)2HPO4 (mixed in the molar ratio of 2:3:2); (3) ErPO4 and K3PO4 (mixed in the molar ratio of 1:1). After the mechanical processing (see “Experimental” section), the above compounds were sintered at different temperatures for different time intervals, which were followed by cooling to room temperature. Phase composition of each sinter was checked by X-ray powder diffraction. A synthesis procedure which consisted in sintering an equimolar mixture of ErPO4 and K3PO4 at 1000 °C for 24 h was accepted as the most advantageous one. This procedure yielded a monoclinic modification of β-K3Er(PO4)2. Attempts to obtain a high-temperature modification α by means of quenching-in-ice failed.
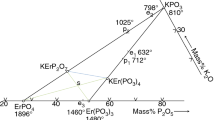
Next, the thermal characteristics of K3Er(PO4)2 phosphate was investigated, including thermal stability, melting point, polymorphism. Three endothermic effects were found on the DTA-heating curve (Fig. 2a). Two successive effects, almost overlapping, occurred at high temperatures; the corresponding temperatures were 1355 ± 3 and 1365 ± 3 °C. DTA-heating examination of ErPO4-rich samples of the ErPO4–K3PO4 system (Fig. 3a) was helpful to determine the phase transitions which the effects were related to (Fig. 1). The DTA curves of those samples showed an endothermic effect with corresponding temperatures between 1350 ± 3 and 1358 ± 3 °C. Based on this, it has been determined that:
-
K3Er(PO4)2 phosphate is unstable at high temperatures; it melts incongruently at 1355 ± 3 °C with the formation of solid ErPO4 and a melt richer in K3PO4,
-
the 1365 °C endothermic effect was connected with the liquidus curve.
A temperature of 420 ± 1.5 °C corresponded to the third endothermic effect shown by the DTA-heating curve of K3Er(PO4)2 phosphate. This effect was ascribed to the β/α-K3Er(PO4)2 transformation. Enthalpy of the transformation, as determined on the basis of calorimetric analysis (DSC, Fig. 2b), amounts to 9.45 ± 1.89 kJ mol−1. According to Ushakov et al. [19], the β/α transition occurs at a temperature of 436.4 °C, and the accompanying thermal effect is larger and amounts of 15.20 ± 3.99 kJ mol−1.
The FTIR spectrum of obtained β-K3Er(PO4)2 at 1000 °C (Fig. 4) displays the characteristic bands of M I3 Ln(PO4)2 orthophosphates [20]. The main absorption bands are observed at 407–413, 540–620, 938, and 970–1120 cm−1. The bands at 407–413 and 540–620 cm−1 are due to the symmetric ν 2 and asymmetricν 4 deformation modes of the PO4 3− group, respectively. The band at 938 cm−1 corresponds to the symmetric stretching ν 1 of PO4 3− group and the intensive bands at 970–1,120 cm−1 to the asymmetric stretching vibrations ν 3 of the P–O bands.
The following two series of samples for investigation were prepared due to the conditions under which the K3Er(PO4)2 compound was formed and, too, the need to have the samples in the equilibrium state:
-
unequimolar mixtures of ErPO4 and K3Er(PO4)2 in the ErPO4-rich part of the system (in the composition range of 0–44.7 wt% K3PO4,
-
unequimolar mixtures of K3Er(PO4)2 and K3PO4 in the K3PO4-rich part of the system (i.e., above 44.7 wt% K3PO4).
After the initial mechanical processing (see “Experimental” section), the mixtures were pressed into pellets and solid state sintered at 1000 °C for 24 h. Next, those were slowly cooled to room temperature and the sinters were crushed and thoroughly rubbed. Such preparations were then investigated by DTA of heating and X-ray diffraction.
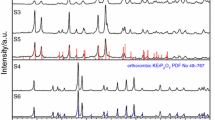
Diffraction patterns of three representative samples of the ErPO4–K3PO4 system (30, 44.7, and 70 wt% K3PO4) are shown in Fig. 5. Comparing the displayed reflections with the ones of the ICDD PDF database for ErPO4 (card no. 1-72-336), for K3PO4 (card no. 20-0921), and K3Er(PO4)2 (card no. 49-1019), indicates that only one intermediate compound is formed in the examined system, namely the K3Er(PO4)2 binary orthophosphate. Reflections present on the diffraction pattern shown in Fig. 5a are typical of ErPO4 and K3Er(PO4)2, whereas those in Fig. 5c are characteristic of K3Er(PO4)2 and K3PO4 phosphates. The diffraction pattern in Fig. 5b demonstrates features of the phase-pure intermediate K3Er(PO4)2 which was self-synthesized; in addition, for comparison, bar graph is given (PDF card no. 49-1019).
Samples of the ErPO4–K3PO4 system were melting at high temperatures (mostly above 1400 °C); hence, prepared sinters were melted in a horizontal furnace. Fusion temperature was read by pyrometer and thus the liquidus curves in Fig. 1, as drawn with mostly dashed lines, have a suggested shape only.
Also, based on the thermoanalytical investigations (distribution of melting point), it has been observed that within the composition range from about 70 to about 95 wt% K3PO4, an area of a limited solubility of the components occurs in the liquid phase below 1520 ± 30 °C. The liquid undergoes separation into two liquid solutions, L1 and L2. An area of unlimited mutual solubility occurs above the temperature 1520 ± 30 °C, which is above point B. Composition of the solution L1 changes along curve BA with decreasing temperature, while that of L2 does along curve BM. The liquid L2 reaches the composition corresponding to the point M (monotectic point) at a temperature of 1420 ± 30 °C. There starts a monotectic transition which proceeds at the constant temperature 1420 ± 30 °C according to the reaction
where L2M denotes the liquid L2 of composition at point M, and L1M does the liquid L1 of composition at point A.
Also, the occurrence of a eutectic is found; it melts at a temperature of 1317 ± 3 °C (Fig. 3b, c). The eutectic point parameters are 58.5 wt% K3PO4, 41.5 wt% ErPO4. Effects connected with the eutectic transformation occur on the DTA-heating curves of samples with the composition range from 45 to about 72 wt% K3PO4 (Figs. 1, 3b, c) Samples with a higher content of potassium orthophosphate do not exhibit those effects. As was mentioned, the K3Er(PO4)2 phosphate melts incongruently at 1355 ± 3 °C. Endothermic effects accompanying this temperature are present on the DTA-heating curves for ErPO4-rich samples up to 46 wt% K3PO4. Peritectic reaction ends at the latter composition (Fig. 1). Endothermic effects at about 420 ± 1.5 °C, which are connected with the β/α-K3Er(PO4)2 transformation, are observed on the DTA-heating curves for all samples of the composition range from 10 to 85 wt% K3PO4.
Polymorphism of K3PO4 was reexamined earlier (1981 year) in our laboratory by the thermal, dilatometric, and X-ray methods [24]. The sintered and the molten potassium orthophosphate was investigated during heating and cooling. It was discovered that the compound appeared in three polymorphic modifications. The transitions in molten K3PO4 during heating took place at 545 °C (γ/β) and 1060–1150 °C (β/α). Over the temperature range between 1060 and 1150 °C, a mixture of α and β modifications occurred [24]. In the ErPO4–K3PO4 system under discussion, the β/α-K3PO4 transition proceeds only at a temperature of 1060 ± 3 °C. A single endothermic effect is observed on the DTA-heating curves of the melted samples; moreover, it is found for the potassium orthophosphate-rich samples, i.e., the ones containing from 70 to 100 wt% K3PO4. Any heating effects, however, have not been observed, which could be connected with the γ/β-K3PO4 transformation.
Conclusions
The phase diagram of the K3PO4–ErPO4 system was determined in the temperature range 20–1896 °C. In the system one, intermediate compound K3Er(PO4)2 was found. It occurs in two polymorphic forms; transformation β/α-K3Er(PO4)2 proceeds at 420 °C. K3Er(PO4)2 phosphate is unstable at high temperatures and melts incongruently at 1355 °C. The minimum melting temperature is 1317 °C and it corresponds to the binary eutectic K3PO4 + ErPO4 with composition of 58.5 wt% K3PO4, 41.5 wt% ErPO4.
References
Salmon R, Parent C, Vlasse M, Le Flem G. The crystal structure of a new high-Nd-concentration laser material: Na3Nd(PO4)2. Mat Res Bull. 1978;13(5):439–44.
Melnikov PP, Kalinin VB, Erremov VA, Komissarova LN. Double ortho-phosphates of rare-earths(Gd–Lu), yttrium, and scandium with rubidium. Inorg Mater. 1981;17(8):1085–7.
Hong HY-P, Chinn SR. Crystal structure and fluorescence lifetime of potassium neodymium orthophosphate, K3Nd(PO4)2, a new laser material. Mat Res Bull. 1976;11:421–8.
Finke B, Schwarz L, Gürtler P, Kraas M, Joppien M, Becker J. Optical properties of potassium rare earth orthophosphates of the type K3RE(PO4)2. J Lumin. 1994;60–61:975–8.
Schwarz L, Finke B, Kloss M, Rohmann A, Sasum U, Haberland D. Investigations on the electronic structure of double phosphates of the type M3RE(PO4)2 (RE = rare earths, lanthanides). J Lumin. 1997;72–74:257–9.
Schwarz L, Kloss M, Rohmann A, Sasum U, Haberlandl D. Investigations of alkaline rare earth orthophosphates M3RE(PO4)2. J Alloys Compd. 1998;275:93–5.
Morozov VA, Bobylev AP, Gerasimova NV, Kirichenko AN, Mikahailin VV, Pushkina GY, Lazoryak BI, Komissarova LN. Structure and spectral properties of double phosphates and vanadates K3Eu(EO4)2 (E = P, V). Russ J Inorg Chem. 2001;46(5):711–8.
Mikhailik VB, Kraus H, Dorenbos P. Efficient VUV sensitization of Eu3+ emission by Tb3+ in potassium rare-earth double phosphate. Phys Status Solidi R. 2009;3(1):13–5.
Szczygieł I, Macalik L, Radomińska E, Znamierowska T, Mączka M, Godlewska P, Hanuza J. Efficient VUV sensitization of Eu3+ emission by Tb3+ in potassium rare-earth double phosphate. Opt Mater. 2007;29:1192–205.
Ju GF, Hu YH, Chen L, Wang X, Mu ZF, Wu HY, Kang FW. A reddish orange-emitting stoichiometric phosphor K3Eu(PO4)2 for white light-emitting diodes. Opt Laser Technol. 2012;44(1):39–42.
Szuszkiewicz W, Znamierowska T. Phase equilibria in the system YPO4–Na3PO4. Themochim Acta. 1991;188:293–7.
Jungowska W, Znamierowska T. The system LaPO4–K3PO4. J Solid State Chem. 1991;95:265–9.
Czupińska G, Znamierowska T. The System YPO4–K3PO4. J Therm Anal. 1993;39:539–44.
Szczygieł I, Znamierowska T. Phase equilibria in the system CePO4–Na3PO4. J Solid State Chem. 1991;95:260–4.
Znamierowska T, Bandrowski Sz. Investigation of phase equilibria in the system Nd2O3–Na2O–P2O5. Quasibinary section NdPO4–Na3PO4. Pol J Chem. 2006;80:1731–5.
Szczygieł I, Matraszek A, Znamierowska T. Phase equilibria in the Ce2O3–K2O–P2O5 system. J Therm Anal Calorim. 2008;93(3):671–6.
Szczygieł I, Znamierowska T, Mizer D. Phase equilibria in the oxide system Nd2O3–K2O–P2O5. Solid State Sci. 2010;12:1205–10.
Szuszkiewicz W, Radominska E, Znamierowska T, Wilk P. Phase equilibria in the YPO4–Rb3PO4 system. J Therm Anal Calorim. 2012; doi:10.1007/s1097301121727.
Ushakov SV, Navrotskya A, Farmer JM, Boatner LA. Thermochemistry of the alkali rare-earth double phosphates, A3Re(PO4)2. J Mater Res. 2004;19(7):2165–75.
Benarafa L, Rghioui L, Nejjar R, Idrissi MS, Knidiri M, Lorriaux A, Wallart F. Theoretical and experimental analysis of the vibration spectra of rare earth potassium phosphates. Spectrochim Acta. 2005;61:419–30.
Ushakov SV, Helean KB, Navrotsky A, Boatner LA. Thermochemistry of rare-earth orthophosphates. J Mater Res. 2001;16:2623–33.
Hikichi Y, Nomura T. Melting temperatures of monazite and xenotime. J Am Ceram Soc. 1987;70(10):252–3.
Hikichi Y, Ota T, Daimon K, Hattori T, Mizuno M. Thermal, mechanical, and chemical properties of sintered xenotime-type RPO4 (R = Y, Er, Yb, or Lu). J Am Ceram Soc. 1998;81:2216–8.
Znamierowska T. Phase equilibria in the system CaO–K2O–P2O5. Part V. Partial system CaO–K3PO4–K4P2O7. Pol J Chem. 1981;55:747–56.
Hetzel A, Ross SD. X-ray powder data and cell dimensions of some rare earth orthophosphates. J Inorg Nucl Chem. 1967;29:2085–9.
Milligan WO, Mullica DF, Beall GW, Boather LA. The structure of three lanthanide orthophosphates. Inorg Chim Acta. 1983;77:133–6.
Kockelmann W, Schafter W, Will G. Neutron diffraction measurements on ErPO4 and ErVO4. Eur J Solid State Chem. 1991;28:515–8.
Ni Y, Hughes JM, Mariano AN. Crystal chemistry of the monazite and xenotime structures. Am Miner. 1995;80:21–6.
Hoppe R, Seyfert HM. Zur Kenntnis wasserfreier Orthophosphate der hoheren Alkalimetalle: K3PO4, Rb3PO4, Cs3PO4. Z Naturforsch. 1973;28(2):507–8.
Kolsi AW. Stabilisation des formes haute temperature des phosphates Na3PO4 et K3PO4 par des cations divalents. Rev Chim Miner. 1976;13(5):416–21.
Voronin VI, Ponosov YuS, Berger IF, Proskurnina NV, Zubkov VG, Tyutyunnik AP, Bushmeleva SN, Balagurov AM, Sheptyakov DV, Burmakin EI, Shekhtman GSh, Vovkotrub EG. Crystal structure of the low-temperature form of K3PO4. Inorg Mater. 2006;42(8):908–13.
Serra JJ, Coutures J, Rouanet A. Traitements thermiques des orthophosphates de lanthanides LnPO4 et formation de nouveaux composés (oxyphosphates). High Temp High Press. 1976;8:337–41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Piotrowska, D., Znamierowska, T. & Szczygieł, I. Phase equilibria in the ErPO4–K3PO4 system. J Therm Anal Calorim 113, 121–126 (2013). https://doi.org/10.1007/s10973-012-2883-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2883-4