Abstract
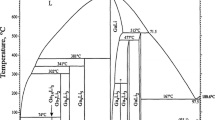
The thermodynamic modeling of the gallium–yttrium binary system was carried out with the help of the CALPHAD (CALculation of PHAse Diagram) method. Ga2Y, GaY, and the three polytypes Ga3Y5 have been treated as stoichiometric compounds while a solution model has been used for the description of the liquid phase and the (Ga) and (Y) solid solutions. The excess term of the Gibbs energy of the solution phases was assessed with the recent exponential temperature dependence of the interaction energies by Kaptay (Calphad 28–2:115–24, 1; Calphad 32–2:338–52, 2; Mat Sci Eng A 495:19–26, 3) and compared with the Redlich–Kister (Ind Eng Chem. 4;40:345) polynomial equation results. The calculations based on the thermodynamic modeling are in good agreement with the phase diagram data and experimental thermodynamic values available in the literature.









Similar content being viewed by others
References
Kaptay G. A new equation for the temperature dependence of the excess Gibbs energy of the solution phase. Calphad. 2004;28–2:115–24.
Kaptay G. A Calphad-compatible method to calculate liquid/liquid interfacial energies in immiscible metallic system. Calphad. 2008;32–2:338–52.
Kaptay G. A unified model for the cohesive enthalpy, critical temperature, surface tension and volume thermal expansion coefficient of liquid metals of bcc, fcc and hcp crystals. Mat Sci Eng A. 2008;495:19–26.
Redlich O, Kister A. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345.
Kanibolotsky DS, Bieloborodova OA, Lisnyak VV. Calorimetric study of enthalpies of mixing in liquid gallium–germanium–yttrium alloys. Thermochim Acta. 2005;438:51.
Kanibolotsky DS, Bieloborodova OA, Lisnyak VV. Examination of enthalpies of mixing in liquid gallium–yttrium alloys by high temperature calorimetry. Thermochim Acta. 2005;437:62–6.
Idbenali M, Servant C. Communication presented at JEEP2011 Saint-Avold, France, published by EDP Science (2011).
[8] Idbenali M., Servant C. Communication presented at Euromat 2011 Montpellier, France.
Lupis CH. Chemical thermodynamics of metals. North-Holland: Prentice-Hall Inc.; 1983.
Kumar KC, Wollants P. Some guides for thermodynamic optimisation of phase diagrams. J Alloys Comp. 2001;320–2:189–98.
Yatsenko SP, Semyannikov AA, Semenov BG, Chuntonov KA. Phase diagrams of rare earth metals with gallium. J Less-Common Met. 1979;64:185–99.
Yatsenko SP. Gallii Vzaimodeistvie s Metallami. Gallium Interaction with Metals. Moscow: Nauka; 1974.
Semenov BG. Thesis, Nauk. SSSR, UNC, Sverdlovsk 1977.
Iandelli AG. Phase diagram. Chim Ital. 1949;79:70.
Buschow KHJ, Hoogenhof WW. Magnetic properties and phase relationships of gadolium–galium compounds. J Less-Common Met. 1976;45–2:309–13.
Yatsenko SP, Anikin Yu A. Izv. Akad. Nauk. SSSR, Met. 1970;4:162.
Gryniv OI, Griniv IA, Gryn YN, Yarmolyuk YP Dop. Akad. Nauk Ukr. RSR, B: geologichni, khimichni ta biologichni Nauk. 1985;5:42.
Kadomatsu H, Sakurai J, Sugamoto J, Fujiwara H. Pressure-induced superconductivity in TbGa6 and DyGa6. Solid State Commun. 1992;82:111–4.
Tagawa Y, Sakurai J, Komura Y, Ishimasa T. Magnetic susceptibility and electrical resistivity of RGa6 (R = rare earth metals). J Less-Common Met. 1986;119:269.
Gryn’ YN, Gladyshewsky RE. Gallides. Metallurgia. Moscow, 1989 (in Russian).
Markiv V.Ya. Belyavina N.M, Dop. Akad. Nauk Ukr., Rep. Nat. Acad. Sci. Ukr., 1992;10:142.
Krachan T, Stel’makhovych B, Kuz’ma Yu. The Y-Ag-Ga system. J Alloys Comp. 2005;386:147–50.
Massalski TB (ed.) Binary alloy phase diagrams, 2nd ed. Materials Park: ASM International; 1990. pp. 1874–1875.
Babu R, Magarajan K, Venugopal V. Standard enthalpies of formation of yttrium gallides by high temperature reaction calorimetry. J Alloys Comp. 2000;311:200–6.
Merker P. Enthalpies of formation of some Ga–Y intermetallic compounds. J Less-Common Met. 1991;69:L23–4.
Yamshchikov LF, Lebedev VA, Nichkov IF, Raspopin SP, Kokoulin OK. Russ J Phys Chem. 1979;53(5):657.
de Boer FR, Boom R, Mattens WC, Miedema AR, Niessen AK. Cohesion in metals, transition metals alloys. Cohesion and structure, vol. 1. Amsterdam: North-Holland; 1988.
Meschel SV, Kleppa OJ. Standard enthalpies of formation of some 4d transition metal gallides by high temperature direct synthesis calorimetry. J Alloys Comp. 2000;297:162–7.
Dinsdale AT. SGTE data for pure elements. Calphad. 1991;15:317–425.
Sundman B, Janson B, Andersson J-O. The Thermo-Calc databank system. Calphad. 1985;9:153–90.
Chen SL, Daniel S, Zhang Z, Chang YA, Oates WA, Schmid Fetzer RJ. On the calculation of multicomponent stable phase diagrams. J Phase Equilib. 2001;22:373.
Zhao J, Corbett JD. R5Ga3 compounds of selected rare earth metals R: structures and properties. J Alloys Comp. 1994;210:1–7.
Shob O, Parthé E. Sc5 and Y5Ga3 with D88 structure. H Acta Cryst. 1964;17:1335–6.
Haszko SE. Rare-earth gallium compounds having the Aluminium-Boride structure. Trans Am Inst Min. 1961;221:201–4.
Dwight AE, Downey JW, Conner RA. Equiatomic compounds of Y and the lanthanides elements with Ga. Acta Cryst. 1967;23:860–2.
Markiv VYa, Belyavina NM, Speka MV. The isothermal section of the phase diagram of the ternary system Y–Ge–Ga at 800°C in the region 33.3 to 100 at.% Y. J. Alloys Compd. 1999;285:167–71.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Idbenali, M., Servant, C. Thermodynamic evaluation of the Ga–Y system. J Therm Anal Calorim 112, 245–253 (2013). https://doi.org/10.1007/s10973-012-2861-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2861-x