Abstract
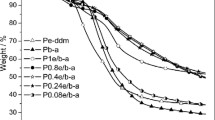
The thermally activated ring-opening polymerization behavior of benzoxazine based on 4,4′-diaminodiphenyl ether was investigated by Fourier transform infrared and differential scanning calorimetry, and the thermal properties of the corresponding polybenzoxazine were studied by dynamic mechanical analysis, thermogravimetry-mass spectrometry, and differential thermal analysis. In the ring-opening polymerization reaction, the C–O–C absorption peak of the oxazine ring at 1,054 cm–1 disappeared first, and the C–N–C absorption intensity of the oxazine ring decreased gradually with time rising. The activation energies of the non-isothermal polymerization are 83.4 and 87.4 kJ mol–1 evaluated with Kissinger and Flynn–Wall–Ozawa methods, respectively. Dynamic mechanical analysis shows the glass transition temperature of the resultant polybenzoxazine is 188 °C. In the thermal degradation, the 10 % mass loss temperature of the polybenzoxazine is 353 °C and the char yield is about 48 % at 800 °C in nitrogen, while 415 °C and close to 0 % at 650 °C in air.
Graphical abstract











Similar content being viewed by others
References
Holly FW, Cope AC. Condensation products of aldehydes and ketones with o-aminobenzyl alcohol and o-hydroxybenzylamine. J Am Chem Soc. 1944;66:1875–9.
Burke WJ, Weatherbee C. 3,4-Dihydro-1,3,2H-benzoxazines. Reaction of polyhydroxybenzenes with N-methylolamines. J Am Chem Soc. 1950;72:4691–4.
Burke WJ, Stephens CW. Monomeric products from the condensation of phenol with formaldehyde and primary amines. J Am Chem Soc. 1952;74:1518–20.
Burke WJ, Murdoch KC, Ec G. Condensation of hydroxyaromatic compounds with formaldehyde and primary aromatic amines. J Am Chem Soc. 1954;76:1677–9.
Burke WJ. 3,4-Dihydro-l,3,2H-benzoxazines. Reaction of p-substituted phenols with N,N-dimethylolamines. J Am Chem Soc. 1949;71:609–12.
Burke WJ, Bishop JL, Glennie ELM, Bauer WN. A new aminoalkylation reaction. Condensation of phenols with dihydro-1,3-aroxazines. J Org Chem. 1965;30:3423–7.
Burke WJ, Glennis ELM, Weatherbee C. Condensation of halophenols with formaldehyde and primary amines. J Org Chem. 1964;29:909–12.
Ning X, Ishida H. Phenolic materials via ring-opening polymerization: synthesis and characterization of bisphenol-A based benzoxazines and their polymers. J Polym Sci Part A. 1994;32:1121–9.
Ning X, Ishida H. Phenolic materials via ring-opening polymerization of benzoxazines: effect of molecular structure on mechanical and dynamic mechanical properties. J Polym Sci Part B. 1994;32:921–7.
Liu YF, Yue ZQ, Gao JG. Synthesis, characterization, and thermally activated polymerization behavior of bisphenol-S/aniline based benzoxazine. Polymer. 2010;51:3722–9.
Liu J, Agag T, Ishida H. Main-chain benzoxazine oligomers: a new approach for resin transfer moldable neat benzoxazines for high performance applications. Polymer. 2010;51:5688–94.
Jia K, Xu MZ, Zhao R, Liu XB. Chemically bonded iron carbonyl for magnetic composites based on phthalonitrile polymers. Polym Int. 2011;60:414–21.
Chen KC, Li HT, Chen WB, Liao CH, Sun KW, Chang FC. Synthesis and characterization of a novel siloxane-imide-containing polybenzoxazine. Polym Int. 2011;60:436–42.
Li SF, Wang LL. Curing behavior of 4,4′-diamonodiphenyl methane-based benzoxazine oligomers/bisoxazoline copolymers and the properties of their cured resins. J Appl Polym Sci. 2006;99:1359–66.
Lin CH, Chang SL, Hsieh CW, Lee HH. Aromatic diamine-based benzoxazines and their high performance thermosets. Polymer. 2008;49:1220–9.
Spontón M, Larrechi MS, Ronda JC, Galià M, Cádiz V. Synthesis and study of the thermal crosslinking of bis(m-aminophenyl) methylphosphine oxide based benzoxazine. J Polym Sci Part A. 2008;46:7162–72.
Andronescu C, Gârea SA, Deleanu C, Iovu H. Characterization and curing kinetics of new benzoxazine monomer based on aromatic diamines. Thermochim Acta. 2012;530:42–51.
Chozhan CK, Alagar M, Gnanasundaram P. Synthesis and characterization of 1,1-bis(3-methyl-4-hydroxy phenyl)-cyclohexane polybenzoxazine–organoclay hybrid nanocomposites. Acta Mater. 2009;57:782–94.
Lin CH, Lin HT, Sie JW, Hwang KY, Tu AP. Facile, one-pot synthesis of aromatic diamine-based phosphinated benzoxazines and their flame-retardant thermosets. J Polym Sci Part A. 2010;48:4555–66.
Lin CH, Chang SL, Shen TY, Shih YS, Lin HT, Wang CF. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym Chem. 2012;3:935–45.
Chang SL, Lin CH. Facile, one-pot synthesis of aromatic diamine-based benzoxazines and their advantages over diamines as epoxy hardeners. J Polym Sci Part A. 2010;48:2430–7.
Qia HM, Ren H, Pan GY, Zhuang YQ, Huang FR, Du L. Synthesis and characteristic of polybenzoxazine with phenylnitrile functional group. Polym Adv Technol. 2009;20:268–72.
Agag T, Jin L, Ishida H. A new synthetic approach for difficult benzoxazines: preparation and polymerization of 4,4′-diaminodiphenyl sulfone-based benzoxazine monomer. Polymer. 2009;50:5940–4.
Ishida H, Rodriguez Y. Curing kinetics of a new benzoxazine-based phenolic resin by differential scanning calorimetry. Polymer. 1995;36:3151–8.
Russell VM, Koenig JL, Low HY, Ishida H. Study of the characterization and curing of benzoxazines using 13C solid-state nuclear magnetic resonance. J Appl Polym Sci. 1998;70:1413–25.
Wang YX, Ishida H. Cationic ring-opening polymerization of benzoxazines. Polymer. 1999;40:4563–70.
Wang YX, Ishida H. Synthesis and properties of new thermoplastic polymers from substituted 3,4-dihydro-2H-1,3-benzoxazines. Macromolecules. 2000;33:2839–47.
Dunkers J, Ishida H. Vibrational assignments of 3-alkyl-3,4-dihydro-6-methyl-2H-1,3-benzoxazines in the fingerprint region. Spectrochim Acta. 1995;51A:1061–74.
Kim HJ, Brunovska Z, Ishida H. Synthesis and thermal characterization of polybenzoxazines based on acetylene-functional monomers. Polymer. 1999;40:6565–73.
Su YC, Yei DR, Chang FC. The kinetics of B-a and P-a type copolybenzoxazine via the ring opening process. J Appl Polym Sci. 2005;95:730–7.
Agag T, Takeichi T. Preparation, characterization, and polymerization of maleimidobenzoxazine monomers as a novel class of thermosetting resins. J Polym Sci Part A. 2006;44:1424–35.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. J Polym Sci Part B. 1966;4:323–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Liu YF, Zhang J, Liu Z, Li ZH, Yue ZQ. Thermally activated polymerization behavior of bisphenol-S/methylamine-based benzoxazine. J Appl Polym Sci. 2012;124:813–22.
Ishida H, Rodriguez Y. Catalyzing the curing reaction of a new benzoxazine based phenolic resin. J Appl Polym Sci. 1995;58:1751–60.
Acknowledgments
This study was financially supported by the Natural Science Foundation of Hebei University (2011YY06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Li, Z., Zhang, J. et al. Polymerization behavior and thermal properties of benzoxazine based on 4,4′-diaminodiphenyl ether. J Therm Anal Calorim 111, 1523–1530 (2013). https://doi.org/10.1007/s10973-012-2480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2480-6