Abstract
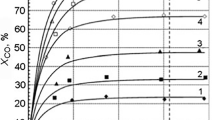
Experiments were performed on calcium oxide, using water vapor with N2 or CO2 as carrier gases, between 40 and 70 °C. A initial experiment was performed with water vapor in the presence of N2 to elucidate the possible hydroxylation process produced by water vapor exclusively. On the other hand, when CO2 was used as carrier gas the CaO reactivity changed, producing different hydrated, hydroxylated, and carbonated phases. On the basis of these results and the fact that under dry conditions CO2 is not absorbed on CaO at T < 70 °C, a possible CaO–H2O–CO2 reaction mechanism was proposed, where CaO superficial hydroxylation process seems to play a very important role during the CO2 capture. Finally, a kinetic analysis was produced to compare the temperature and humidity relative influence on the whole process.







Similar content being viewed by others
References
Song CS. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today. 2006;115:2–32.
Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL. Global climate change and terrestrial net primary production. Nature. 1993;363:234–40.
Han KK, Zhou Y, Chun Y, Zhu JH. Efficient MgO-based mesoporous CO2 trapper and its performance at high temperature. J Hazard Mater. 2012;203–204:341–7.
Choi S, Drese JH, Jones CW. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem. 2009;2:796–854.
Zhao HY, Cao Y, Lineberry Q, Pan WP. Evaluation of CO2 adsorption capacity of solid sorbents. J Therm Anal Calorim. 2011;106:199–205.
Lin PC, Huang CW, Hsiao CT, Teng H. Magnesium hydroxide extracted from a magnesium-rich mineral for CO2 sequestration in a gas–solid system. Environ Sci Technol. 2008;42:2748–52.
Lee SC, Chae HJ, Lee SJ, Choi BY, Yi CK, Lee JB, Ryu CK, Kim JC. Development of regenerable MgO-based sorbent promoted with K2CO3 for CO2 capture at low temperatures. Environ Sci Technol. 2008;42:2736–41.
Torres-Rodríguez DA, Pfeiffer H. Thermokinetic analysis of the MgO surface carbonation process in the presence of water vapor. Thermochim Acta. 2011;516:74–8.
Li L, Wen X, Fu X, Wang F, Zhao N, Xiao FK, Wei W, Sun YH. MgO/Al2O3 sorbent for CO2 capture. Energy Fuels. 2010;24:5773–80.
Anthony EJ. Solid looping cycles: a new technology for coal conversion. Ind Eng Chem Res. 2008;47:1747–53.
Dean CC, Blamey J, Florin NH, Al-Jeboori MJ, Fennell PS. The calcium looping cycle for CO2 capture from power generation, cement manufacture and hydrogen production. Chem Eng Res Des. 2011;89:836–55.
Abanades JC. The maximum capture efficiency of CO2 using a carbonation/calcination cycle of CaO/CaCO3. Chem Eng J. 2002;90:303–6.
Abanades JC, Álvarez D. Conversion limits in the reaction of CO2 with lime. Energy Fuels. 2003;17:308–15.
Filitz R, Kierzkowska AM, Broda M, Müller CR. Highly efficient CO2 sorbents: development of synthetic, calcium-rich dolomites. Environ Sci Technol. 2012;46:559–65.
Broda M, Kierzkowska AM, Müller CR. Application of the sol–gel technique to develop synthetic calcium-based sorbents with excellent carbon dioxide capture characteristics. ChemSusChem. 2012;5:411–8.
Chrissafis K. Multicyclic study on the carbonation of CaO using different limestones. J Therm Anal Calor. 2007;89:525–9.
Prigiobbe V, Hänchen M, Werner M, Baciocchi R, Mazzotti M. Mineral carbonation process for CO2 sequestration. Energy Procedia. 2009;1:4885–90.
Jarvis K, Carpenter RW, Windman T, Kim Y, Nunez R, Alawheh F. Reaction mechanisms for enhancing mineral sequestration of CO2. Environ Sci Technol. 2009;43:6314–9.
Xiong Y, Lord AS. Experimental investigations of the reaction path in the MgO–CO2–H2O system in solutions with various ionic strengths, and their applications to nuclear waste isolation. Appl Geochem. 2008;23:1634–59.
Pfeiffer H, Ávalos-Rendón T, Lima E, Valente JS. Thermochemical and cyclability analyses of the CO2 absorption process on a Ca/Al layered double hydroxide. J Environ Eng. 2011;137:1058–65.
Lindén I, Backman P, Brink A, Hupa M. Influence of water vapor on carbonation of CaO in the temperature range 400–550 °C. Ind Eng Chem Res. 2011;50:14115–20.
Martínez I, Grasa G, Murillo R, Arias B, Abanades JC. Evaluation of CO2 carrying capacity of reactivated CaO by hydration. Energy Fuels. 2011;25:1294–301.
Arias B, Grasa G, Abanades JC, Manovic V, Anthony EJ. The effect of steam on the fast carbonation reaction rates of CaO. Ind Eng Chem Res. 2012;51:2478–82.
Manovic V, Anthony EJ. Carbonation of CaO-based sorbents enhanced by steam addition. Ind Eng Chem Res. 2010;49:9105–10.
Dobner S, Sterns L, Graff RA, Squires AM. Cyclic calcination and recarbonation of calcined dolomite. Ind Eng Chem Process Des Dev. 1977;16:479–86.
Sing K. The use of nitrogen adsorption for the characterisation of porous materials. Coll Surf A. 2001;187–188:3–9.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. London: Wiley; 1999.
Miller FA, Wilkins CH. Infrared spectra and characteristic frequencies of inorganic ions. Anal Chem. 1952;24:1253–94.
Acknowledgements
This study was financially supported by the project SENER-CONACYT 150358. Authors thank A. Tejeda and M. A. Canseco for technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez-Rueda, A., Pfeiffer, H. Thermogravimetric analysis of the water vapor addition during the CaO carbonation process at moderate temperatures (40–70 °C). J Therm Anal Calorim 111, 1385–1390 (2013). https://doi.org/10.1007/s10973-012-2477-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2477-1