Abstract
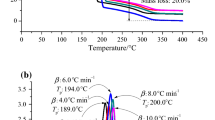
When above certain temperature limits, lauroyl peroxide is an unstable material. If the thermal source cannot be properly governed during any stage in the preparation, manufacturing process, storage or transport, runaway reactions may inevitably be induced immediately. In this study, the influence of runaway reactions on its basic thermal characteristic was assessed by evaluating thermokinetic parameters, such as activation energy (E a) and frequency factor (A) by thermal activity monitor III (TAM III). This was achieved under five isothermal conditions of 50, 60, 70, 80, and 90 °C. Vent sizing package 2 (VSP2) was employed to determine the maximum pressure (P max), maximum temperature (T max ), maximum self-heating rate ((dT dt −1)max), maximum pressure rise rate ((dP dt −1)max), and isothermal time to maximum rate ((TMR)iso) under the worst case. Results of this study will be provided to relevant plants for adopting best practices in emergency response or accident control.










Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor/m3 mol−1 s−1
- C v :
-
Heat capacity under constant volume/J kg−1
- E a :
-
Activation energy/kJ mol−1
- f(α):
-
Kinetic function depends on conversions/dimensionless
- k :
-
Reaction rate constant/variables
- k 0 :
-
Frequency factor/(l/mol) n1 × (1/s)
- m :
-
Total mass of reactant/g
- n :
-
Reaction order/dimensionless
- P :
-
Thermal power or heat production rate/W = J s−1
- Q :
-
Heat/J
- Q :
-
Heat flow/J g−1
- SADT:
-
Self-accelerating decomposition temperature/°C
- T :
-
Absolute temperature/K
- T max :
-
Maximum temperature during overall reaction/°C
- T 0 :
-
Exothermic onset temperature/°C
- T f :
-
Final temperature/°C
- T NR :
-
Temperature of no return/°C
- t :
-
Time/sec
- TMRiso :
-
Isothermal time to maximum rate/min, hr, or day
- ∆H d :
-
Heat of decomposition/J g−1
- ∆H iso :
-
Heat of decomposition under isothermal condition/J g−1
- dα dt −1 :
-
Reaction rate/s−1
- (dP dt −1)max :
-
Maximum pressure rise rate/bar min−1
- (dT dt −1)max :
-
Maximum self-heating rate/°C min−1
- Φ:
-
Thermal inertial/dimensionless
References
Moad G, Solomon DH. The chemistry of free radical polymerization. Oxford: Pergamon Press; 1995.
Bevington JC, Hunt BJ. The use of stabilized radicals with monomers and lauroyl peroxide. Eur Polym J. 2004;40:103–8.
Guillet JE, Gilmer JC. Decomposition of lauroyl, decanoyl, and octanoyl peroxides in solution. Can J Chem. 1969;47:4405–8.
Kotoyori T. The self-accelerating decomposition temperature (SADT) of solids of the quasi-autocatalytic decomposition type1. J Hazard Mater. 1999;A64:1–19.
Chu YC, Chen JR, Tseng JM, Tsai LC, Shu CM. Evaluation of runaway thermal reactions of di-tert-butyl peroxide employing calorimetric approaches. J Therm Anal Calorim. 2011;106:227–34.
Liang YC, Jhu CY, Wu SH, Shen SJ, Shu CM. Evaluation of adiabatic runaway reaction of methyl ethyl ketone peroxide by DSC and VSP2. J Therm Anal Calorim. 2011;106:173–7.
Tseng JM, Shu CM. Isothermal kinetic evaluation of methyl ethyl ketone peroxide mixed withacetone by TAM III tests. Thermochim Acta. 2010;45–48:507–8.
Lin WH, Wu SH, Shiu GY, Shieh SS, Shu CM. Self-accelerating decomposition temperature (SADT) calculation of methyl ethyl ketone peroxide using an adiabatic calorimeter and model. J Therm Anal Calorim. 2009;2:245–51.
FAI/06-90. VSP2 User's Manual & Test Methods (Software Version 4.3). Fauske & Associates, LLC, Burr Ridge, IL. 2006.
Askonas CF, Burelbach JP, Leung JC (2000) The versatile VSP2: a tool for adiabatic thermal analysis and vent sizing applications. North American Thermal Analysis Society, 28th Annual Conference, vol 1. Orlando, pp 4–6.
Li XR, Koseki H. Thermal decomposition of liquid organic peroxide. J Loss Prev Process Ind. 2005;18:460–4.
Li XR, Koseki H. SADT prediction of autocatalytic material using isothermal calorimetry analysis. Thermochim Acta. 2004;423:77–82.
Li XR, Koseki H. Thermal decomposition kinetic of reactive solids based on isothermal calorimetry measurements. J Therm Anal Calorim. 2006;85(3):637–42.
Tseng JM, Liu MY, Chen SL, Horng JJ, Hwang WT, Gupta JP, Shu CM. Runaway effects of nitric acid on methyl ethyl ketone peroxide by TAM III tests. J Therm Anal Calorim. 2006;96:789–93.
Fu ZM, Li XR, Koseki H, Mok YS. Evaluation on thermal hazard of methyl ethyl ketone peroxide by using adiabatic method. J Loss Prev Process Ind. 2003;16:389–93.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. J Thermochim Acta. 1980;37:1–30.
Tseng JM, Chang RH, Horng JJ, Chang MK, Shu CM. Thermal hazard evaluation for methyl ethyl ketone peroxide mixed with inorganic acids. J Therm Anal Calorim. 2006;83:57–62.
Chi JH, Wu SH, Shu CM. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J Hazard Mater. 2009;171:1145–9.
Su CH, Wu SH, Shen SJ, Shiue GY, Wang YW, Shu CM. Thermal characteristics and regeneration analyses of adsorbents by differential scanning calorimetry and scanning electron microscope. J Therm Anal Calorim. 2009;96:765–9.
Lee RP, Hou HY, Tseng JM, Chang MK, Shu CM. Reactive incompatibility of DTPB mixed with two acid solutions. J Therm Anal Calorim. 2008;93:269–74.
Liaw HJ, Yur CC, Lin YF. A mathematical model for predicting thermal hazard data. J Loss Prev Process Ind. 2000;13:499–507.
Huang CC, Peng JJ, Wu SH, Hou HY, You ML, Shu CM. Effects of cumene hydroperoxide on phenol and acetone manufacturing by DSC and VSP2. J Therm Anal Calorim. 2010;102:579–85.
Acknowledgements
The authors are grateful to the members of the Process Safety & Disaster Prevention Laboratory in Taiwan for technical assistance and for providing valuable comments. Furthermore, the authors deeply appreciate the financial support by National Science Council (NSC) of Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, JM., You, ML., Chu, YC. et al. Evaluation of thermal hazard for lauroyl peroxide by VSP2 and TAM III. J Therm Anal Calorim 109, 1237–1243 (2012). https://doi.org/10.1007/s10973-012-2350-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2350-2