Abstract
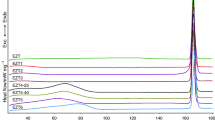
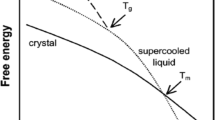
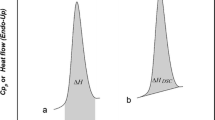
Morphological and thermodynamic transitions in drugs as well as their amorphous and crystalline content in the solid state have been distinguished by thermal analytical techniques, which include dielectric analysis (DEA), differential scanning calorimetry (DSC), and macro-photomicrography. These techniques were used successfully to establish a structure versus property relationship with the United States Pharmacopeia standard set of active pharmaceutical ingredient (API) drugs. A distinguishing method is the DSC determination of the amorphous and crystalline content which is based on the fusion properties of the specific drug and its recrystallization. The DSC technique to determine the crystalline and amorphous content is based on a series of heat and cool cycles to evaluate the drugs ability to recrystallize. To enhance the amorphous portion, the API is heated above its melting temperature and cooled with liquid nitrogen to −120 °C (153 K). Alternatively a sample is program heated and cooled by DSC at a rate of 10 °C min−1. DEA measures the crystalline solid and amorphous liquid API electrical ionic conductivity. The DEA ionic conductivity is repeatable and differentiates the solid crystalline drug with a low conductivity level (10−2 pS cm−1) and a high conductivity level associated with the amorphous liquid (106 pS cm−1). The DSC sets the analytical transition temperature range from melting to recrystallization. However, analysis of the DEA ionic conductivity cycle establishes the quantitative amorphous and crystalline content in the solid state at frequencies of 0.10–1.00 Hz and to greater than 30 °C below the melting transition as the peak melting temperature. This describes the “activation energy method.” An Arrhenius plot, log ionic conductivity versus reciprocal temperature (K−1), of the pre-melt DEA transition yields frequency dependent activation energy (E a, J mol−1) for the complex charging in the solid state. The amorphous content is inversely proportional to the E a where the E a for the crystalline form is higher and lower for the amorphous form with a standard deviation of ±2%. There was a good agreement between the DSC crystalline melting, recrystallization, and the solid state DEA conductivity method with relevant microscopic evaluation. An alternate technique to determine amorphous and crystalline content has been established for the drugs of interest based on an obvious amorphous and crystalline state identified by macro-photomicrography and compared to the conductivity variations. This second “empirical method” correlates well with the “activation energy” method.











Similar content being viewed by others
References
Riga A, Judovits L. Materials characterization by dynamic and modulated thermal analytical techniques. West Conshohocken: ASTM. Special technical publication; 2011.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48:27–42.
Jójárt-Laczkovich O, Szabó-Révész P. Amorphization of a crystalline active pharmaceutical ingredient and thermo analytical measurements on this glassy form. J Therm Anal Calorim. 2009. doi:10.1007/s10973-009-0530-5.
Cui Y. A material science perspective of pharmaceutical solids. Int J Pharm. 2007;339:3–18.
Hancock BC. Disordered drug delivery: destiny, dynamics and the Deborah number. J Pharm Pharmacol. 2002;54:737–46.
Hancock BC, Zografi G. Characteristic and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86:1–12.
Keré J, Sréié S. Thermal analysis of glassy pharmaceuticals. Thermochim Acta. 1995;248:81–95.
Angell CA. The old problems of glass and the glass transition, and the many new twists. Proc Natl Acad Sci USA. 1995;92:6675–82.
Byrn S, Pfeiffer R, Ganey M, Hoi berg C, Poochkian G. Pharmaceutical solids: a strategic approach to regulatory consideration. Pharm Res. 1995;12:945–54.
Vippagunta Sr, Brittain HG, Grant DJW. Crystalline solids. Adv Drug Deliv Rev. 2001;48:3–26.
Venkatesh GM, Barnett ME, Fordjour CO, Galop M. Detection of low levels of the amorphous phase in the crystalline materials by thermally stimulated current spectrometry. Pharm Res. 2001;18:98–103.
Shah B, Kaumanu VK, Bansal AK. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J Pharm Sci. 2006;95:1641–65.
Lappalainen Minna, Karppinen Maarit. Techniques of differential scanning calorimetry for quantification of low contents of amorphous phases. J Therm Anal Calorim. 2010;102:171–80.
Hancock BC, Parks M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm Res. 2000;17:397–403.
Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Deliv Rev. 2004;56:335–47.
Schmitt EA, Law D, Zhang GGZ. Nucleation and crystallization kinetics of hydrated amorphous lactose above the glass transition temperature. J Pharm Sci. 1999;88:291–6.
Hancock BC, Shamblin SL, Zografi G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperature. Pharm Res. 1995;12:799–806.
Seyer JJ, Luner PE, Kemper MS. Application of diffuse reflectance near infrared spectroscopy for the determination of crystallinity. J Pharm Sci. 2000;89:1305–16.
Hendrickson BA. Characterization of calcium fenoprofen 1. Powder dissolution rate and degree of crystallinity. Int J Pharm. 1990;60:243–52.
Duddu SP, Sokoloski TD. Dielectric analysis in the characterization of amorphous pharmaceutical solids. 1. Molecular mobility in poly (vinylpyrrolidine)–water systems in the glassy state. J Pharm Sci. 1995;84:773–6.
Gustafson C, Lennholm H, Iversen T, Nystrom C. Comparison of solid state NMR and isothermal microcalorimetry in the assessment of the amorphous component of lactose. Int J Pharm. 1998;174:243–52.
Taylor LS, Zografi G. The quantitative analysis of crystallinity using FT-Raman spectroscopy. Pharm Res. 1998;15:755–61.
Black DB, Lovering EG. Estimation of the degree of crystallinity in digoxin by X-ray and Infrared methods. J Pharm Sci. 1997;29:684–7.
Riga Alan T, Alexander Kenneth S. Electrical conductivity analysis/dielectric analysis differentiates physical-chemical properties of drugs and excipients. Am Pharm Rev. 2005;8(6):45–51.
Thakur SS, Maheswaram MPK, Mantheni DR, Kaza L, Perera I, Ball DW, Moran J, Riga AT. Solid–state mechanical properties of crystalline drugs and excipients: new data substantiate discovered dielectric viscoelastic characteristics. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-18559-0.
Mantheni DR, Maheswaram MPK, Sobhi HF, Perera NI, Riga AT, Ellen Matthews M, Alexander K. Solid state studies of drugs and chemicals by dielectric and calorimetric analysis. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1423-y.
Sharma M, Yashonath S. Correlation between conductivity and diffusivity and activation energy in amorphous solids. J Chem Phys. 2008;129:144103.
Matthews ME, Atkinson I, Presswala L, Najjar O, Gerhardstein N, Wei R, Rye E, Riga AT. Dielectric classification of d- and l-amino acids by thermal and analytical methods. J Therm Anal Calorim. 2008;93:281–7.
Presswala L, Matthews ME, Atkinson I, Najjar O, Gerhardstein N, Moran J, Wei R, Riga AT. The discovery of bound and unbound waters in crystalline amino acids revealed by thermal analysis. J Therm Anal Calorim. 2008;93:295–300.
Matthews ME, Riga AT. Effects of thermal history on solid state and melting behavior of amino acids. J Therm Anal Calorim. 2009;96(3):673–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maheswaram, M.P., Mantheni, D., Perera, I. et al. Characterization of crystalline and amorphous content in pharmaceutical solids by dielectric thermal analysis. J Therm Anal Calorim 111, 1987–1997 (2013). https://doi.org/10.1007/s10973-011-2140-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2140-2