Abstract
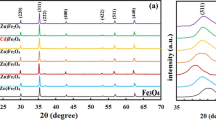
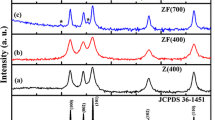
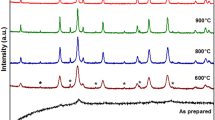
Zn0.5Ni0.5Fe2(C2O4)3·6H2O was synthesized by solid-state reaction at low heat using ZnSO4·7H2O, NiSO4·6H2O, FeSO4·7H2O, and Na2C2O4 as raw materials. The spinel Zn0.5Ni0.5Fe2O4 was obtained via calcining Zn0.5Ni0.5Fe2(C2O4)3·6H2O above 773 K in air. The Zn0.5Ni0.5Fe2(C2O4)3·6H2O and its calcined products were characterized by thermogravimetry and differential scanning calorimetry (TG/DSC), Fourier transform FT-IR, X-ray powder diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectrometer (EDS), and vibrating sample magnetometer (VSM). The result showed that Zn0.5Ni0.5Fe2O4 obtained at 1073 K had a saturation magnetization of 86.7 emu g−1. The thermal process of Zn0.5Ni0.5Fe2(C2O4)3·6H2O experienced three steps, which involved the dehydration of the six crystal water molecules at first, and then decomposition of Zn0.5Ni0.5Fe2(C2O4)3 into Zn0.5Ni0.5Fe2O4 in air, and at last crystallization of Zn0.5Ni0.5Fe2O4. Based on KAS equation, and OFW equation, the values of the activation energies associated with the thermal process of Zn0.5Ni0.5Fe2(C2O4)3·6H2O were determined to be 126.02 ± 23.93, and 259.76 ± 18.67 kJ mol−1 for the first, and second thermal process steps, respectively. Dehydration of the six waters of Zn0.5Ni0.5Fe2(C2O4)3·6H2O is multi-step reaction mechanisms. Decomposition of Zn0.5Ni0.5Fe2(C2O4)3 into Zn0.5Ni0.5Fe2O4 could be simple reaction mechanism, probable mechanism function integral form of thermal decomposition of Zn0.5Ni0.5Fe2(C2O4)3 is determined to be g(α) = [−ln(1 − α)]4.







Similar content being viewed by others
References
Zhang K, Holloway T, Pradhan AK. Magnetic behavior of nanocrystalline CoFe2O4. J Magn Magn Mater. 2011;323:1616–22.
Mozaffari M, Eghbali Arani M, Amighian J. The effect of cation distribution on magnetization of ZnFe2O4 nanoparticles. J Magn Magn Mater. 2010;322:3240–4.
Satyanarayana L, Madhusudan Reddy K, Manorama SV. Nanosized spinel NiFe2O4: a novel material for the detection of liquefied petroleum gas in air. Mater Chem Phys. 2003;82:21–6.
Gonsalves LR, Mojumdar SC, Verenkar VMS. Synthesis and characterization of Co0.8Zn0.2Fe2O4 nanoparticles. J Therm Anal Calorim. 2011;104:869–73.
Gawas UB, Verenkar VMS, Mojumdar SC. Synthesis and characterization of Ni0.6Zn0.4Fe2O4 nano-particles obtained by auto catalytic thermal decomposition of carboxylato-hydrazinate complex. J Therm Anal Calorim. 2011;104:879–83.
Chandradass J, Kim KH. Solvent effects in the synthesis of MgFe2O4 nanopowders by reverse micelle processing. J Alloys Compd. 2011;509:59–62.
Costa ACFM, Silva VJ, Cornejo DR, Morelli MR, Kiminami RHGA, Gama L. Magnetic and structural properties of NiFe2O4 ferrite nanopowder doped with Zn2+. J Magn Magn Mater. 2008;320:370–2.
Gul IH, Ahmed W, Maqsood A. Electrical and magnetic characterization of nanocrystalline Ni–Zn ferrite synthesis by co-precipitation route. J Magn Magn Mater. 2008;320:270–5.
Anil Kumar PS, Shrotri JJ, Kulkami SD, Deshpande CE, Date SK. Low temperature synthesis of Ni0.8Zn0.2Fe2O4 powder and its characterization. Mater Lett. 1996;27:293–6.
Jiang CT, Liu RJ, Shen XQ, Zhu L, Song FZ. Ni0.5Zn0.5Fe2O4 nanoparticles and their magnetic properties and adsorption of bovine serum albumin. Powder Technol. 2011;211:90–4.
Mouallem-Bahout M, Bertrand S, Peña O. Synthesis and characterization of Zn1-xNixFe2O4 spinels prepared by a citrate precursor. J Solid State Chem. 2005;178:1080–6.
Wu WW, Hou SY, Wu XH, Li SS, Liao S, Nan WN. Synthesis of nanocrystalline spinel Zn0.5Ni0.5Fe2O4 via solid-state reaction at low heat. Nonferr Met. 2010;62:18–21 (in Chinese).
Zahi S, Daud AR, Hashim M. A comparative study of nickel–zinc ferrites by sol–gel route and solid-state reaction. Mater Chem Phys. 2007;106:452–6.
Pal M, Brahma P, Chakravorty D, Bhattacharyya D, Maiti HS. Nanocrystalline nickel-zinc ferrite prepared by the glass-ceramic route. J Magn Magn Mater. 1996;164:256–60.
Bid S, Pradhan SK. Characterization of crystalline structure of ball-milled nano-Ni–Zn-ferrite by Rietveld method. Mater Chem Phys. 2004;84:291–301.
Hallynck S, Pourroy G, Vilminot S, Jacquart PM, Autissier D, Vukadinovic N, Pascard H. Synthesis of high aspect ratio of Ni0.5Zn0.5Fe2O4 platelets for electromagnetic devices. Solid State Sci. 2006;8:24–30.
Zhong ZF, Li Q, Zhang YL, Zhong HS, Cheng M, Zhang Y. Synthesis of nanocrystalline Ni–Zn ferrite powders by refluxing method. Powder Technol. 2005;155:193–5.
Uskoković V, Drofenik M, Ban I. The characterization of nanosized nickel-zinc ferrites synthesized within reverse micelles of CTAB/1-hexanol/water microemulsion. J Magn Magn Mater. 2004;284:294–302.
Gubbala S, Nathani H, Koizol K, Misra RDK. Magnetic properties of nanocrystalline Ni–Zn, Zn–Mn, and Ni–Mn ferrites synthesized by reverse micelle technique. Phys B. 2004;348:317–28.
Upadhyay C, Mishra D, Verma HC, Anand S, Das RP. Effect of preparation conditions on formation of nanophase Ni–Zn ferrites through hydrothermal technique. J Magn Magn Mater. 2003;260:188–94.
Costa ACFM, Morelli MR, Kiminami RHGA. Microstructure and magnetic properties of Ni1–xZnxFe2O4 synthesized by combustion reaction. J Mater Sci. 2007;42:779–83.
Wu XH, Wu WW, Cui XM, Liao S. Preparation of nanocrystalline BiFeO3 via a simple and novel method and its kinetics of crystallization. J Therm Anal Calorim. (2011). doi:10.1007/s10973-011-1483-z.
Chrissafis K. Kinetics of thermal degradation of polymers. Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Chen ZP, Chai Q, Liao S, He Y, Wu WW, Li B. Preparation of LiZnPO4·H2O via a novel modified method and its non-isothermal kinetics and thermodynamics of thermal decomposition. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1799-8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH. The ‘temperature integral’—its use and abuse. Thermochim Acta. 1997;300:83–92.
Vlaev L, Nedelchev N, Gyurova K, Zagorcheva M. A comparative study of non-isothermal kinetics of decomposition of calcium oxalate monohydrate. J Anal Appl Pyrolysis. 2008;81:253–62.
Senum GI, Yang RT. Rational approximations of the integral of the Arrhenius function. J Therm Anal. 1977;11:445–7.
Liqing L, Donghua C. Application of iso-temperature method of multiple rate to kinetic analysis. Dehydration for calcium oxalate monohydrate. J Therm Anal Calorim. 2004;78:283–93.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly(tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99:551–61.
Wu XH, Wu WW, Liu C, Li SS, Liao S, Cai JC. Synthesis of layered sodium manganese phosphate via low-heating solid-state reaction and its properties. Chin J Chem. 2010;28:2394–8.
Wu XH, Wu WW, Cui XM, Liao S. Selective self-assembly synthesis of MnV2O6·4H2O with controlled morphologies and study on its thermal decomposition. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1577-7.
Donia AM. Synthesis, identification and thermal analysis of coprecipitates of silver-(cobalt, nickel, copper and zinc) oxalate. Polyhedron. 1997;16:3013–31.
Goel SP, Mehrotra PN. IR and thermal studies on lithium oxomolybdenum (VI) oxalate. J Therm Anal. 1985;30:145–51.
Li FS, Wang HB, Wang L, Wang JB. Magnetic properties of ZnFe2O4 nanoparticles produced by a low-temperature solid-state reaction method. J Magn Magn Mater. 2007;309:295–9.
Wu WW, Hou SY, Liao S, Wang MJ, Wu XH, Li SS. Nanocrystalline spinel NiFe2O4 synthesis by solid-state reaction at low heat. Nonferr Met. 2010;62:39–42 (in Chinese).
Acknowledgements
This study was financially supported by the National Nature Science Foundation of China (Grant no. 21161002) and the Guangxi Nature Science Foundation of China (Grant no. 2011GXNSFA018036).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Li, Y., Zhou, K. et al. Nanocrystalline Zn0.5Ni0.5Fe2O4 . J Therm Anal Calorim 110, 1143–1151 (2012). https://doi.org/10.1007/s10973-011-2027-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-2027-2