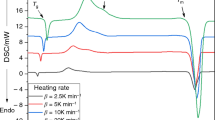
Abstract
In the present study samples of Se100 − x S x has been prepared by conventional melt-quenching technique in the composition range 5 ≤ x ≤ 20 (at.%). The crystallization process in glassy system was investigated under non-isothermal condition using differential scanning calorimetry (DSC) at 5, 10, 15, and 20 °C/min heating rates (ϕ). The DSC traces have been analyzed in terms of activation energy (ΔE c) and Avrami exponent (n) using different models viz. the Starink, Flynn–Wall–Ozawa, the Friedman–Ozawa, Kissinger–Akahira–Sunose equations. The composition dependence on the glass transition temperature (T g), the crystallization temperature (T c), and the peak temperatures (T p) of the samples were also determined. The analysis shows that the incorporation of sulfur content has a strong influence on the crystallization mechanism for the Se–S glassy system.








Similar content being viewed by others
References
Starink MJ. The analysis of Al-based alloys by calorimetry: quantitative analysis of reactions and reaction kinetics. Int Mater Rev. 2004;49:191–226.
Ozawa T. Temperature control modes in thermal analysis. Pure Appl Chem. 2000;72:2083–99.
Ahamad MN, Vaish R, Varma KBR. Calorimetric studies on 2TeO2–V2O5 glasses. J Therm Anal Calorim. 2011;105:239–43.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Liu L, Zhi FW, Chen L. A kinetic study of the non-isothermal crystallization of a Zr-based bulk metallic glass. Chin Phys Lett. 2002;19:1483–6.
Vazquez J, Barreda DGG, Lopez-Alemany PL, Villares P, Jimenez-Garay R. Crystallization of Ge0.08Sb0.15Se0.77 glass studied by DSC. J Noncryst Solids. 2004;345 and 346:142–7.
Wang J, Liu YJ, Tang CY, Liu LB, Zhou HY, Jin ZP. Thermodynamic description of the Au–Ag–Ge ternary system. Thermochim Acta. 2011;512:240–6.
Surinach S, Baro MD, Clavaguera-Mora MT, Clavaguera N. Kinetic study of isothermal and continuous heating crystallization in GeSe2–GeTe–Sb2Te3 alloy glasses. J Noncryst Solids. 1983;58:209–17.
Kotkata MF, El-Fouly H, El-Behay AZ, El-Wahab LA. Transport studies of S–Se amorphous semiconductors. Mater Sci Eng. 1983;60:163–71.
Mahmoud EA. Crystallization kinetics of amorphous S20Se80. Therm Anal Calorim. 1990;36:1481–6.
Kotkata MF, Ayad FM, El-Mously MK. Photo-effect on crystallization kinetics of amorphous selenium doped with sulphur. J Noncryst Solids. 1979;33:13–22.
El-Shazly O, Ramadan T, El-Anany N, Mataweh HA, El-Wahidy E. Calorimetric studies of chalcogenide glasses in the system Se–S. Eur Phys J Appl Phys. 2001;13:161–5.
Dimitrov D, Tzocheva D, Kovacheva D. Calorimetric study of amorphous Sb–Se thin films. Thin Solids Films. 1998;323:79–84.
Mahadevan S, Giridhar A, Singh AK. Calorimetric measurements on As–Sb–Se glasses. J Noncryst Solids. 1986;88:11–34.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Kujirai T, Akahira T. Effect of temperature on the deterioration of fibrous insulation materials. Sci Pap Inst Phys Chem Res. 1925;2:223–52.
Akahira T, Sunose T. Joint convention of four electrical institutes. Res Rep Chiba Inst Technol. 1971;16:22–31.
Starink MJ. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim Acta. 1996;288:97–104.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57:217–52.
Ozawa T. Estimation of activation energy by isoconversion methods. Thermochim Acta. 1992;203:159–65.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick direct method for the determination of activation energy from thermogravimetric data. J Polym Sci. 1966;B4:323–7.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J Polym Sci. 1964;C6:183–95.
Ozawa T. Applicability of Friedman plot. J Therm Anal Calorim. 1986;31:547–51.
Malek J, Cernoskova E, Svejka R, Sestak J, Van der Plaat G. Crystallization kinetics of Ge0.3Sb1.4S2.7 glass. Thermochim Acta. 1996;280–281:353–61.
Afify N. Calorimetric study on the crystallization of a Se0.8Te0.2 chalcogenide glass. J Noncryst Solids. 1992;142:247–59.
Calventus Y, Surinach S, Baro MD. Crystallization mechanisms of a Se85Te15 glassy alloy. J Phys Condens Matter. 1996;8:927–40.
Suzuki T, Arai Y, Ohishi Y. Crystallization processes of Li2O–Ga2O3–SiO2–NiO system glasses. J Noncryst Solids. 2007;353:36–43.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Ozawa T. Kinetics of non-isothermal crystallization. Polymer. 1971;12:150–8.
Vazquez J, Wagner C, Villares P, Jimenez-Garay R. Glass transition and crystallization kinetics in Sb0.18As0.34Se0.48 glassy alloy by using non-isothermal techniques. J Noncryst Solids. 1998;235–237:548–53.
Hsiao A, McHenry ME, Laughlin DE, Kramer MJ, Ashe C, Ohkubo T. The thermal, magnetic, and structural characterization of the crystallization kinetics of Fe88Zr7B4Cu1, an amorphous soft magnetic ribbon. IEEE Trans Magn. 2002;38:3039–44.
Acknowledgements
The author M. A. Majeed Khan is thankful to the members of King Abdullah Institute for Nanotechnology for their kind support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musahwar, N., Khan, W., Husain, M. et al. Non-isothermal kinetic analysis on the crystallization process in Se–S glassy system. J Therm Anal Calorim 110, 823–829 (2012). https://doi.org/10.1007/s10973-011-1972-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1972-0