Abstract
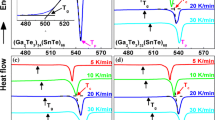
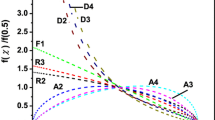
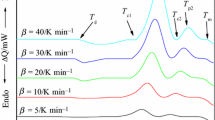
Silver-containing chalcogenide glasses are well-known candidates for technical applications as well as for the fundamental studies. In the present report, we have studied the kinetics of glass/crystal phase transformation in novel synthesized glasses of STSA system. For this, purpose, differential scanning calorimetric technique has been employed at four different heating rates (5, 10, 15, 20 K min−1). This paper explores the thermal analysis of calorimetric data using both advanced iso-conversional methods for the determination of effective activation energy as a function of extent of crystallization and classical non-isothermal methods for the determination of over-all crystallization activation energy. Iso-conversional methods, such as Kissinger–Akahira–Sunose method and Flynn–Wall–Ozawa method, have been used to study the variation of activation energy of crystallization and other kinetic parameters with extent of crystallization. Non-isothermal methods (Kissinger method, Augis–Bennett method, and Matusita–Sakka method) have been used to determine over-all activation energy of crystallization and other significant parameter of thermally activated crystallization. We have also explained the composition dependence of various kinetic parameters.










Similar content being viewed by others
References
Goryunova NA, Kolomiets BT. Glassy semiconductors IX. Glass-formation in compound chalcogenides based on arsenic sulfide and selenide. Solid State Phys. 1960;2:280–3.
Ohta T. Phase-change optical memory promotes the DVD optical disc. J Opto Adv Mater. 2001;3:609–26.
Popescu M. Disorder chalcogenide optoelectronic materials: phenomena and application. J Optoelectron Adv Mater. 2005;7:2189–210.
Hamann HF, O’Boyle M, Martin YC, Rooks M, Wickramasinghe HK. Ultra-high-density phase-change storage and memory. Nat Mater. 2006;5:383–7.
Wuttig M, Yamada N. Phase-change materials for rewriteable data storage. Nat Mater. 2007;6:824–33.
Lencer D, Salinga M, Grabowski B, Hickel T, Neugebauer J, Wuttig M. A map for phase-change materials. Nat Mater. 2008;7:972–7.
Rocca J, Fontana M, Arcondo B. Simulation of non-volatile memory cell using chalcogenide glasses. J Alloys Compd. 2012;536S:S516–21.
Toupin P, Brilland L, Boussard-Pledel C, Bureau B, Mechin D, Adam JL, Troles J. Comparison between chalcogenide glass single index and micro structured exposed-core fibers for chemical sensing. J Non-Cryst Solids. 2013;377:217–9.
Xu H, He Y, Wang X, Nie Q, Zhang P, Xu T, Dai S, Zhang X, Tao G. Preparation of low-loss Ge15Ga10Te75 chalcogenide glass for far-IR optics applications. Infrared Phys Technol. 2014;65:77–82.
Lucas P, Garrett JC, Jiang S, Luo T, Yang Z. Chalcogenide glass fibers: optical window tailoring and suitability for bio-chemical sensing. J Opt Mater. 2015;47:530–6.
Zakery A, Elliott SR. Optical properties and applications of chalcogenide glasses: a review. J Non-Cryst Solids. 2003;330:1–12.
MehtaN KumarA. Some new observations on activation energy of crystal growth for thermally activated crystallization. J Phys Chem B. 2016;120:1175–82.
Vyazovkin S. Computational aspects of kinetic analysis: part C. The ICTAC kinetics project—the light at the end of the tunnel? Thermochim Acta. 2000;355:155–63.
Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand. 1956;57:217–21.
Kissinger HE. Reaction kinetics in differential thermal analysis. J Anal Chem. 1957;29:1702–6.
Akahira T, Sunose T. Method of determining activation deterioration constant of electrical insulating materials. Res Rep Chiba Inst Technol. 1971;16:22–31.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Japan. 1965;38:181–6.
Ozawa T. Kinetics of non-isothermal crystallization. Polymer. 1971;12:150–8.
Flynn JH, Wall LA. Thermal analysis of polymer by thermogravimetric analysis. J Res Natl Bur Stand A. 1966;70:487–523.
Doyle CD. Kinetic analysis of thermogravimetric data. J Appl Polym Sci. 1961;5:285–92.
Doyle CD. Series approximations to the equation of thermogravimetric data. Nature. 1965;207:290–301.
Wanjun T, Donghua C. An integral method to determine variation in activation energy with extent of conversion. Thermochim Acta. 2005;443:72–6.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Starink MJ. Comments on precipitation kinetics of Al–1.12Mg2Si–0.35Si and Al–1.07Mg2Si–0.33Cu alloys. J Alloys Compd. 2007;443:L4–6.
Johnson WA, Mehl RF. Reaction kinetics in processes of nucleation and growth. Trans Am Inst Min (Mettal) Eng. 1939;135:416–42.
Avrami M. Kinetics of phase change I. J Phys Chem. 1939;7:1103–12.
Avrami M. Kinetics of phase change II. J Phys Chem. 1940;8:212–24.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Matusita K, Sakka S. Kinetic study of the crystallization of glass by differential scanning calorimetry. Phys Chem Glasses. 1979;20:81–4.
Matusita K, Sakka S. Kinetic study on non-isothermal crystallization of glass by thermal analysis. Bull Inst Chem Res. 1981;59:159–71.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Augis JA, Bennett JE. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal. 1978;13:283–92.
Cui S, Boussard-Pledel C, Lucas J, Bureau B. Te-based glass fiber for far infrared sensing up to 16 μm. Opt Express. 2014;22:21253–62.
Bureau B, Boussard-Pledel C, Lucas P, Zhang X, Lucas J. Forming glasses from Se and Te. Molecules. 2009;14:4337–50.
Cui S, Chahal R, Boussard-Pledel C, Nazabal V, Doualan JL, Troles J, Lucas J, Bureau B. From selenium to tellurium based glass optical fibers for infrared spectroscopies. Molecules. 2013;18:5373–88.
Kumar H, Mehta N, Singh K. Calorimetric studies of thermal crystallization in glassy Se80-xTe20Snx (0 ≤ x ≤ 10) alloys. Phys Scr. 2011;83:065602.
Kumar H, Mehta N. Thermal characterization of glassy Se78−xTe20Sn2Pbx (0 ≤ x ≤ 6) alloys for phase change optical recording technique. Glass Phys Chem. 2013;39:490–8.
Kumar H, Mehta N. Kinematical studies of thermal crystallization in glassy Se78−xTe20Sn2Bix (0 ≤ x ≤ 6) alloys. J Adv Phys. 2013;2:163–9.
Frumar M, Wagner T. Ag doped chalcogenide glasses and their applications. Curr Opin Solid Stat Mater Sci. 2003;7:117–26.
Vyazovkin S. A unified approach to kinetic processing of non-isothermal data. Int J Chem Kinet. 1996;28:95–101.
Vyazovkin S, Sbirrazzuoli N. Isoconversional analysis of calorimetric data on non-isothermal crystallization of a polymer melt. J Phys Chem B. 2003;107:882–8.
Turnbull D, Fisher JC. Rate of nucleation in condensed systems. J Chem Phys. 1949;17:71–3.
Fusong J, Xu Y, Jiang M, Gan F. Film preparation and structure analysis of optical recording domains of amorphous Ge–Sb–Te. J Non-Cryst Solids. 1995;184:51–6.
Chou LH, Kuo MC. Thin InSb films—a candidate for multiple recording. J Appl Phys. 1995;77:1964–8.
Chiba R, Funakoshi N. Crystallization of vacuum deposited Te–Se–Cu alloy film. J Non-Cryst Solids. 1988;105:149–54.
Petford-Long AK, Doole RC, Afonso CN, Solis J. In situ studies of the crystallization kinetics in Sb–Ge films. J Appl Phys. 1995;77:607–13.
Matusita K, Konatsu T, Yokota R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J Mater Sci. 1984;19:291–6.
Acknowledgements
One of us, NM is grateful to the Board of Research in Nuclear Sciences (BRNS), Mumbai, India for providing financial support under DAE Research Award for Young Scientists (Scheme No. 2011/20/37P/02/BRNS) and Banaras Hindu University for providing financial support under DST-Purse programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivastava, A., Chandel, N. & Mehta, N. Calorimetric studies of crystallization for multi-component glasses of Se–Te–Sn–Ag (STSA) system using model-free and model-fitting non-isothermal methods. J Therm Anal Calorim 128, 907–914 (2017). https://doi.org/10.1007/s10973-016-6019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-6019-0