Abstract
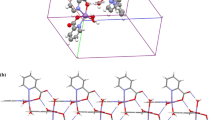
In this study, we analyzed influence of the type of the syntheses used: hydrothermal and non-hydrothermal on pyridine-2,3-dicarboxylic acid (2,3pydcH2) coordination fashion. Two manganese(II) complexes: [Mn(H2O)3(2,3pydc)] n (1) and [Mn(H2O)6][Mn(2,3pydcH)3]2 (2) were successfully synthesized from the non-hydrothermal reaction system containing organic ligand and different Mn(II) salts. The received complexes have been prepared and characterized by spectroscopic (IR, Raman), structural (X-ray single crystal), and thermogravimetric methods. The results of the crystal study give some evidence that ligand exhibits various topological structures and interesting properties. Pyridine-2,3-dicarboxylic acid acts as monodicarboxylate N,O-chelating anion (complex 2) or a doubly deprotonated three-dentate-N,O,O′ dicarboxylate ion (complex 1). In the [Mn(H2O)6][Mn(2,3pydcH)3]2 the coordination geometry around Mn(1) ion can be considered as being distorted octahedron {MnN3O3}. The Mn(2) cation possesses the same coordination polyhedron (octahedral). We have also analyzed influence of furnace atmosphere on the thermal behavior and the kind of final product. The sample of (1) decomposes in four stages in N2 (368–1073 K) and the final residue is MnO2. The thermogram of (2) exhibits three main distinct decomposition steps (383–973 K). A residue of MnO is remained. In both air and nitrogen atmosphere, Mn(II) complexes (1) and (2) keep unchanged over all steps of decomposition. Only the final residues are different (Mn2O3 are formed). The course of pyrolysis and molecular structure of the complexes lead to the same conclusion about the strength of metal–ligand bonds. On the basis of the above results, it is concluded that the thermal stability of the manganese(II) compounds is slightly different.






Similar content being viewed by others
References
Kang Y, Zhang J, Li ZJ, Cheng JK, Yao YG. Syntheses, structures, and photoluminescent properties of four d10 metal-quinolinato coordination polymers with similar rod-like SBUs. Inorg Chim Acta. 2006;359:2201–9.
Li M, Xiang J, Yuan L, Wu S, Chen S, Sun J. Syntheses, structures and photoluminescence of three novel coordination polymers constructed from dimeric d10 metal units. Cryst Growth Des. 2006;6:2036–40.
Yang H, Zhang ZH, Guo JH, Lu YC. Hydrothermal and crystal structure of a coordination polymer: [Ni(pda)(H2O)3]n (pda = pyridine-2, 3-dicarboxylate). Chin J Struct Chem. 2006;25:689–93.
Lush SF, Shen FM. Poly[(μ 4-pyridine-2,3-dicarboxylato)-lead(II)]. Acta Cryst. 2011;Sect. E67:m163–4.
Du ZX, Li JX. Catena-Poly[[[diaquamanganese(II)]-μ3-pyridine-2,3-dicarboxylato-κ4 N,O 2:O 3:O 3′] dihydrate]. Acta Cryst. 2008;Sect. E64:m1295–6.
Gharagozlou M, Langer V, Nemati A. Hexaaquazinc(II) bis[tris(3-carboxypyridine-2-carboxylato)zincate(II)]. Acta Cryst. 2010;Sect. E66:m1606–7.
Barszcz B, Hodorowicz M, Jabłońska-Wawrzycka A, Masternak J, Nitek W, Stadnicka K. Comparative study on Cd(II) and Ca(II) model complexes with pyridine-2,3-dicarboxylic acid: Synthesis, crystal structure and spectroscopic investigation. Polyhedron. 2010;29:1191–200.
Hao L, Mu C, Kong B. catena-Poly[[[triaquacopper(II)]-μ-pyridine-2,3-dicarboxylato-κ3 N,O 2:O 3] monohydrate]. Acta Cryst. 2008;Sect. E64:m1229.
Jaber F, Cherbonnier F, Faure R. Preparation and crystal structure of tetraaqua-bis(hydrogenopyridine-2,3-(dicarboxylate)bis(pyridine-2,3-dicarboxylate)hexa silver(I) [Ag6(C7H4NO4)2(C7H3NO4]n. Polyhedron. 1996;15:2909–13.
Patrick BO, Stevens CL, Storr A, Thompson RC. Structural and magnetic properties of three copper(II) pyridine-2, 3-dicarboxylate coordination polymers incorporating the same chain motif. Polyhedron. 2003;22:3025–35.
Yin H, Liu SX. Copper and zinc complexes with 2,3-pyridinedicarboxylic acid or 2,3-pyrazinedicarboxylic acid; Polymer structures and magnetic properties. J Mol Struct. 2009;918:165–73.
Han ZB, Ma Y, Sun ZG, You WS. Hydrothermal synthesis, crystal structure and photoluminescent properties of a novel 3-D coordination polymer [Cd2(PYDC)2(H2O)]n (pydc = pyridine-2, 3-dicarboxylate). Inorg Chem Commun. 2006;9:844–7.
Zhang CX, Ma CB, Wang M, Chen CN. Synthesis and crystal structure of a new three-dimensional coordination polymer: [Mn(2, 3-pdc)(H2O)]n (2, 3-pdc = pyridine-2,3-dicarboxylate). Chin J Struct Chem. 2008;27:1370–4.
Li LJ, Li Y. Hydrothermal synthesis and crystal structure of a novel 2-D coordination polymer [Mn2(pdc)2(H2O)3] n ∙2n H2O (pdc = pyridine-2,3-dicarboxylate). J Mol Struct. 2004;694:199–203.
Yen CH, Chen CY, Shiu KB. Hydrothermal synthesis and X-ray structural characterization of manganese, nickel, and cadmium coordination polymers containing 2,3-pyridinedicarboxylate as multidentate ligands. J Chin Chem Soc. 2007;54:903–10.
Okabe N, Miura J, Shimosaki A. A hydrated cobalt(II) complex of quinolinic acid: trans-[Co(C7H4NO4)2(H2O)2]. Acta Cryst. 1996;Sect. C52:1610–2.
Aghabozorg H, Sadr-khanlou E, Soleimannejad J, Adams H. Diaqua bis(3-carboxypyridine-2-carboxylato-κ2 N,O 2)zinc(II). Acta Cryst. 2007;Sect. E63:m1769.
Xiang JF, Li M, Wu SM, Yuan LJ, Sun JT. Diaqua bis(pyridine-2,3-dicarboxylato)copper(II). Acta Cryst. 2006;Sect. E62:m1122–3.
Singh WM, Jali BR, Das B, Baruah JB. Synthesis, characterization, and reactivity of zinc carboxylate complexes of 2,3-pyridine dicarboxylic acid and (3-oxo-2, 3-dihydro-benzo[1, 4]oxazin-4-yl)acetic acid. Inorg Chim Acta. 2011;372:37–41.
Turner DR, Batten SR. catena-Poly[[copper(II)-bios(μ-3-carboxypyridine-2-carboxylato)-κ3 N,O 2:O 3;κ3 O 3:N,O 2] methanol disolvate]. Acta Cryst. 2007;Sect. E63:m452–4.
Das B, Boudalis AK, Baruah JB. Selective adenine/cytosine cations in one-dimensional coordination polymers of manganese (II) and copper (II) 2,3-pyridinedicarboxylates. Inorg Chem Commun. 2010;13:1244–8.
Nonius COLLECT. Delft: Nonius BV; 1997–2000.
Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation. Methods Enzymol. 1997;276:307.
Altomare A, Cascarano G, Giacovazzo C, Guagliardi C, Burla MC, Palidori G, Camalli M. SIR92-a program for automatic solution of crystal structures by direct methods. J Appl Cryst. 1994;27:435.
Scheldrick GM. SHELXL-97, Program for Crystal Structure Refinement. Germany: University of Göttingen; 1997.
Brandenburg K, Putz H. Diamond-crystal and molecular structure visualization crystal impact. Rathausgasse 30, version 3.1f. Bonn: GbR; 1997–2000.
Faruggia L. WinGX suite for small-molecule single-crystal crystallography. J Appl Cryst. 1999;32:837–8.
Deacon GB, Phillips RJ. Relationship between the carbon-oxygen stretching frequencies of carboxylate and the type of carboxylate coordination. Coord Chem Rev. 1980;33:227–50.
Barszcz B, Masternak J, Surga W. Thermal properties of Ca(II) and Cd(II) complexes of pyridinedicarboxylates. Correlation with crystal structures. J Therm Anal Calorim. 2010;101:633–9.
Nakamoto K. Infrared spectra of inorganic and coordination compounds. 6th ed. Hoboken: Wiley; 2009.
Robert V, Lemercier G. A combined experimental and theoretical study of carboxylate coordination modes: A structural probe. J Am Chem Soc. 2006;128:1183–7.
Brzyska W, Jusko IA. J Therm Anal Calorim. 2004;76:823–8.
Vairam S, Premkumor T, Govindarajan S. J Therm Anal Calorim. 2010;101:979–85.
Suzuki Y, Muraishi K, Ito H. Thermal decomposition of manganese(II) dicarboxylate anhydrides in various atmospheres. Thermochim Acta. 1995;258:231–41.
Yeşilel OZ, Ölmez H. Spectrothermal studies of 1,10-phenantroline complexes of Co(II), Ni(II), Cu(II) and Cd(II) orotates. J Therm Anal Calorim. 2006;86:211–6.
Çolak AT, Akduman D, Yeşilel OZ, Büyükgüngör O. Pyridine-2,3-dicarboxylic acid complexes of nickel(II) with 2,2′-bipyridine and 1,10-phenantroline coligands; syntheses, crystal structures, spectroscopic and thermal studies. Transition Met Chem. 2009;34:861–8.
Rzączyńska Z, Kula A, Sienkiewicz-Gromiuk J, Szybiak A. Synthesis, spectroscopic and thermal studies of 2,3-naphthalenedicarboxylates of rare earth elements. J Therm Anal Calorim. 2011;103:275–81.
Powder Diffraction File, JCPDS: ICDD, 1601 Park Lane, Swarthmore, PA 19081, Data 1990, File No 24-735.
Rzączyńska Z, Ostasz A, Pikus S. Thermal properties of rare earth elements complexes with 1,3,5-benzenetricarboxylic acid. J Therm Anal Calorim. 2005;82:347–51.
Ferenc W, Walków-Dziewulska A. Comparison of some properties of 2,3- and 3,5-dimethoxybenzoates of light lanthanides. J Therm Anal Calorim. 2003;74:511–9.
Powder Diffraction File, JCPDS: ICDD, 1601 Park Lane, Swarthmore, PA 19081, Data 1990, File No 7-230.
Powder Diffraction File, JCPDS: ICDD, 1601 Park Lane, Swarthmore, PA 19081, Data 1990, File No 6-540.
Acknowledgements
The authors are grateful to Dr. Wiesław Surga and MSc Joanna Masternak for help during the thermal work and XRD investigations. European Union Project 8.2.1/POKL/2009 supported this work (M. Zienkiewicz) partly. The opportunity of making the Raman spectra in the Structural Laboratory of the Jan Kochanowski University is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jabłońska–Wawrzycka, A., Zienkiewicz, M., Hodorowicz, M. et al. Thermal behavior of manganese(II) complexes with pyridine-2,3-dicarboxylic acid. J Therm Anal Calorim 110, 1367–1376 (2012). https://doi.org/10.1007/s10973-011-1971-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1971-1