Abstract
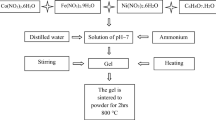
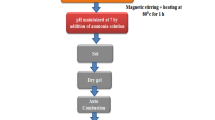
Nickel ferrite powders were synthesized by thermal decomposition of the precursors obtained in the redox reaction between the mixture of Ni(NO3)2·6H2O and Fe(NO3)3·9H2O with polyalcohol: 1,4-butanediol, polyvinyl alcohol and also with their mixture. During this reaction the primary C–OH groups were oxidized at –COOH, while secondary C–OH groups at C=O groups. The carboxylic groups formed coordinate to the present Ni(II) and Fe(III) cations leading to carboxylate type compounds, further used as precursors for NiFe2O4. These precursors were characterized by thermal analysis and FT-IR spectrometry. All precursors thermally decomposed up to 350 °C leading to nickel ferrite weakly crystallized. By annealing at higher temperatures, nanocrystalline nickel ferrite powders were obtained, as resulted from XRD. SEM images have evidenced the formation of nanoparticulate powders; these powders present magnetic properties characteristic to the oxidic system formed by magnetic nanoparticles.











Similar content being viewed by others
Abbreviations
- 1,4BG:
-
1,4-butanediol
- PVA:
-
Polyvinyl alcohol
References
Liu XM, Yang G, Fu SY. Mass synthesis of nanocrystalline spinel ferrites by a polymer-pyrolysis route. Mat Sci Eng C. 2007;27:750–5.
Bucko MM, Haberko K. Hydrothermal synthesis of nickel ferrite powders, their properties and sintering. J Eur Ceram Soc. 2007;27:723–7.
Wang L, Ren J, Wang Y, Liu X, Wang Y. Controlled synthesis of magnetic spinel-type nickel ferrite nanoparticles by the interface reaction and hydrothermal crystallization. J Alloy Compd. 2010;490:656–60.
Zhao DL, Zeng XW, Xia QS, Tang JT. Preparation and coercivity and saturation magnetization dependence of inductive heating property of Fe3O4 nanoparticles in an alternating current magnetic field for localized hyperthermia. J. Alloys Compd. 2009;469:215–8.
Gill CS, Long W, Jones CW. Magnetic nanoparticle polymer brush catalysts: alternative hybrid organic/inorganic structures to obtain high, local catalyst loadings for use in organic transformations. Catal Lett. 2009;131:425–31.
Gadkari AB, Shinde TJ, Vasambekar PN. Ferrite gas sensors. (IEEE) Sens J. 2011;11:849–61.
Boonchom B, Maensiri S. Non-isothermal decomposition kinetics of NiFe2O4 nanoparticles synthesized using egg white solution route. J Therm Anal Calorim. 2009;97:879–84.
Popescu SA, Vlazan P, Notingher PV, Novaconi S, Grozescu I, Bucur A, Sfirloaga P. Synthesis of Ni ferrite powders by coprecipitation and hydrothermal methods. J Optoelectron Adv Mater. 2011;13:260–2.
Shafi KVPM, Ulman A, Yan X, Yang NL, Estourne C, White H, Rafailovich M. Sonochemical synthesis of functionalized amorphous iron oxide nanoparticles. Langmuir. 2001;17:5093–7.
Bensebaa F, Zavaliche F, L’Ecuyer P, Cochrane RW, Veres TJ. Microwave synthesis and characterization of co-ferrite nanoparticles. J Colloid Interface Sci. 2004;277:104.
Lee Y, Lee J, Bae CJ, Park JG, Noh HJ, Park JH, Hyeon T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv Funct Mater. 2005;15:503.
Gingasu D, Mindru I, Patron L, Stoleriu S. Synthesis of lithium ferrites from polymetallic carboxylates. J Serb Chem Soc. 2008;73:979–88.
Mooney KE, Nelson JA, Wagner MJ. Superparamagnetic cobalt ferrite nanocrystals synthesized by alkalide reduction. Chem Mater. 2004;16:3155.
Liu XM, Fu SY, Huang J. Synthesis and magnetic characterization of novel CoFe2O4–BiFeO3 nanocomposites. Mater Sci Eng B. 2005;121:255–60.
Misra RDK, Kale A, Kooi BJ, Hosson JTM. Some aspects of nanocrystalline nickel and zinc ferrites processed using microemulsion technique. Mater Sci Technol. 2003;19:1617–21.
Liu Xian-Ming, Fu SY, Xiao HM, Huang CJ. Synthesis of nanocrystalline spinel CoFe2O4 via a polymer-pyrolysis route. Phys B. 2005;370:14–21.
Manova E, Tsoncheva T, Paneva D, Rehspringer JL, Tenchev K, Mitov I, Petrov L. Synthesis characterization and catalytic properties of nanodimensional nickel ferrite/silica composites. Appl Catal A: General. 2007;317:34–42.
Saha SK, Pathak A, Pramanic P. Low temperature preparation of fine particles of mixed oxides. J Mater Sci Lett. 1995;14:35–7.
Ari M, Miller KJ, Marinkovic BA, Jardim PM, Avillez R, Rizzo F, White MA. Rapid synthesis of the low thermal expansion phase of Al2Mo3O12 via a sol–gel method using polyvinyl alcohol. J Sol-Gel Sci Technol. doi:10.1007/s10971-010-2364-9.
Sen A, Pramanik P. Preparation of nano-sized calcium, magnesium and zinc chromite powder through metalo-organic precursor solution. J Mater Synth Process. 2002;10:107–11.
Stefănescu M, Stoia M, Stefănescu O, Barvinschi P. Obtaining of Ni0.65Zn0.35Fe2O4 nanoparticles at low temperature starting from metallic nitrates and polyols. J Therm Anal Calorim. 2010;99:459–64.
Ştefănescu M, Stoia M, Dippong T, Ştefănescu O, Barvinschi P. Preparation of Co x Fe3−x O4 oxydic system starting from metal nitrates and propanediol. Acta Chim Slov. 2009;56:379–85.
Ştefănescu M, Ştefănescu O, Stoia M, Lazau C. Thermal decomposition of some metal-organic precursors Fe2O3 nanoparticles. J Therm Anal Calorim. 2007;881:27–32.
Gonsalves LR, Mojumdar SC, Verenkar VMS. Synthesis of cobalt nickel ferrite nanoparticles via autocatalytic decomposition of the precursor. J Therm Anal Calorim. 2010;100:789–92.
Joint Committee on Powder Diffraction Standards-International Center for Diffraction Data. Swarthmore, 1993.
Huo J, Wei M. Characterization and magnetic properties of nanocrystalline nickel ferrite synthesized by hydrothermal method. Mater Lett. 2009;63:1183–4.
Acknowledgements
This study was partially supported by the strategic grant POSDRU/21/1.5/G/13798 inside POSDRU Romania 2007–2013, co-financed by the European Social Fund – Investing in People and by the strategic grant POSDRU/88/1.5/S/50783, Project ID 50783 (2009), co-financed by the European Social Fund – Investing in People, within the Sectoral Operational Programme Human Resources Development 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoia, M., Barvinschi, P., Tudoran, L.B. et al. Synthesis of nanocrystalline nickel ferrite by thermal decomposition of organic precursors. J Therm Anal Calorim 108, 1033–1039 (2012). https://doi.org/10.1007/s10973-011-1903-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1903-0