Abstract
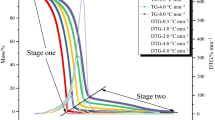
Organic peroxides (OPs) and inorganic peroxides (IPs) are usually employed as an initiator for polymerization, a source of free radicals, a hardener, and a linking agent in low density polyethylene (LDPE), polyvinyl chloride (PVC), controlled-rheology polypropylene (CR-PP), and styrene industries. Worldwide, due to their unstably reactive natures, OPs and IPs have caused many serious thermal explosions and runaway reaction incidents. This study was conducted to elucidate its essentially hazardous characteristics. To analyze the runaway behavior of OPs and IPs in the traditional process, thermokinetic parameters including heat of decomposition (ΔH d ), exothermic onset temperature (T 0), self-accelerating decomposition temperature (SADT), time to maximum rate (TMR), critical temperature (T c ), etc., were measured by calorimetric approaches involving differential scanning calorimetry (DSC), vent sizing package 2 (VSP2), and calculation method. Safety and health handling information of hazardous materials and toxic substances is noted in material safety data sheets (MSDS) and was applied to analyze in process safety management (PSM) in the chemical industries, but MSDS are not providing important handling indicators concerning the SADT, TMR, T c , etc. In view of loss prevention, more useful indicators must be provided in the sheets or guide book.








Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor, sec−1 M1–n
- C p :
-
Liquid specific heat at constant pressure, kJ kg−1 °C−1
- C 0 :
-
Initial concentration, mol L−1
- E a :
-
Activation energy, kJ mol−1
- K :
-
Pre-exponential factor, s−1
- M :
-
Mass of reactant, g
- P max :
-
Maximum pressure during overall reaction, psig
- Q :
-
Calorific capacity, J g−1.
- \( \mathop Q\limits^{ \bullet } \) :
-
heat flow, W g−1
- R :
-
Gas constant, 8.314 J mol−1 K−1
- S :
-
Wetted surface area, m2
- SADT:
-
Self-accelerating decomposition temperature, °C
- T :
-
Temperature, °C
- T A :
-
Final adjusted temperature, K
- T A0 :
-
Initial adjusted temperature, K
- T f :
-
Final temperature, °C
- T M :
-
Final measured temperature, K
- T max :
-
Maximum temperature during overall reaction, °C
- T M0 :
-
Initial measured temperature, K
- T NR :
-
Temperature of no return, °C
- T wall :
-
Temperature on the wall, °C
- TMRad :
-
Time to maximum rate under adiabatic system, min, h
- U :
-
Heat transfer coefficient, kJ min−1 m−2 K−1
- a :
-
Vessel wetted surface area, m2
- k i :
-
Rate at stage i, s−1
- m :
-
Mass of reactor, kg
- n :
-
Order of reaction, dimensionless
- α:
-
Degree of conversion, dimensionless
- β:
-
Heating rate, °C min−1
- λ:
-
Heat conductivity, J ms K−1
- \( \varphi \) :
-
Thermal inertia, dimensionless
- ∆H d :
-
Heat of decomposition, J g−1
- (dT dt −1):
-
Self-heating rate, °C min−1
- (dT dt −1)A :
-
Actual self-heating rate, °C min−1
References
Chen KY, Wu SH, Wang YW, Shu CM. Runaway reaction and thermal hazards simulation of cumene hydroperoxide by DSC. J Loss Prev Process Ind. 2008;21:101–9.
Wu SH, Wang YW, Wu TC, Hu WN, Shu CM. Evaluation of thermal hazards for dicumyl peroxide by DSC and VSP2. J Therm Anal Calorim. 2008;93(1):189–94.
Yang D, Koseki H, Hasegawa K. Predicting the self-accelerating decomposition temperature (SADT) of organic peroxide based on non-isothermal decomposition behavior. J Loss Prev Process Ind. 2003;16:411–6.
Miyaka A, Yamada N, Ogawa T. Mixing hazard evaluation of organic peroxides with other chemicals. J Loss Prev Process Ind. 2005;18:380–3.
Duh YS, Wu XH, Kao CS. Hazard ratings for organic peroxides. Process Safety Progress. 2008;27(2):89–99.
Li XR, Koseki H. SADT prediction of autocatalytic material using isothermal calorimetry analysis. Thermochimica acta. 2005;431:113–6.
Lin WH, Wu SH, Shiu GY, Shieh SS, Shu CM. Self-accelerating decomposition temperature (SADT) calculation of methyl ethyl ketone peroxide using an adiabatic calorimeter and model. J Therm Anal Calorim. 2009;95(2):645–51.
Liao CC, Wu SH, Su TS, Shyu ML, Shu CM. Thermokinetic evaluation and simulations for the polymerization of styrene in the presence of various inhibitor concentrations. J Therm Anal Calorim. 2006;85(1):65–71.
You ML, Liu MY, Wu SH, Chi JH, Shu CM. Thermal explosion and runaway reaction simulation of lauroyl peroxide by DSC tests. J Therm Anal Calorim. 2009;96:777–82.
Semonov NN. Some problems of chemical kinetics and reactivity Part II. London: Pergamon Press; 1959.
Luo KM, Lu KT, Hu KH. The critical condition and stability of exothermic chemical reaction in a non-isothermal reactor. J Loss Prev Process Ind. 1997;10(3):141–50.
Luo KM, Hu KH. The stability of toluene mononitration in reaction calorimeter reactor. J Loss Prev Process Ind. 1998;11:413–21.
Luo KM, Lin SH, Chang JG, Lu KT, Chang CT, Hu KH. The critical runaway condition and stability criterion in a Phenol-Formaldehyde reaction. J Loss Prev Process Ind. 2000;13:91–108.
Luo KM, Chang JG, Lin SH, Chang CT, Yeh TF, Hu KH, Kao CS. The criterion of critical runaway and stable Temperatures in cumene hydroperoxide reaction. J Loss Prev Process Ind. 2001;14:229–39.
Maria G, Heinzle E. Kinetic system identification by using short-cut techniques in early safety assessment of chemical processes. J Loss Prev Process Ind. 1998;11:187–206.
Chang RH, Tseng JM, Jehng JM, Shu CM, Hou HY. Thermokinetic model simulations for methyl ethyl ketone peroxide contaminated with H2SO4 or NaOH by DSC and VSP2. J Therm Anal Calorim. 2006;83(1):57–62.
Chang RH, Shu CM, Duh YS, Jehng JM. Calorimetric studies on the thermal hazard of methyl ethyl ketone peroxide with incompatible substances. J Hazard Mater. 2007;141:762–8.
Tseng JM, Chang YY, Su TS, Shu CM. Study of thermal decomposition of methyl ethyl ketone peroxide using DSC and simulation. J Hazard Mater. 2007;142:765–70.
Lin YF, Tseng JM, Wu TC, Shu CM. Effects of acetone on methyl ethyl ketone peroxide runaway reaction. J Hazard Mater. 2008;153:1071–7.
Lee RP, Hou HY, Tseng JM, Chang MK, Shu CM. Reactive incompatibility of DTBP mixed with two acid solutions. J Therm Anal Calorim. 2008;93(1):269–74.
Chen JR, Wu SH, Lin SY, Hou HY, Shu CM. Utilization of microcalorimetry for an assessment of the potential for a runaway decomposition of cumene hydroperoxide at low temperatures. J Therm Anal Calorim. 2008;93(1):127–33.
Peng JJ, Wu SH, Hou HY, Lin CP, Shu CM. Thermal hazards evaluation of cumene hydroperoxide mixed with its derivatives. J Therm Anal Calorim. 2009;96:783–7.
Sun J, Li Y, Hasegawa K. A study of self-accelerating decomposition (SADT) using reaction calorimetry. J Loss Prev Process Ind. 2001;14:331–6.
Chou YP, Huang JY, Tseng JM, Cheng SY, Shu CM. Reaction hazard analysis for the thermal decomposition of cumene hydroperoxide in the presence of sodium hydroxide. J Therm Anal Calorim. 2008;93(1):275–80.
Chou YP, Hou HY, Chang RH, You ML, Peng JY, Shu CM. Thermal decomposition of cumene hydroperoxide in the presence of three incompatible substances by isothermal microcalorimetry and high performance liquid chromatography. J Therm Anal Calorim. 2009;96:771–5.
Hou HY, Duh YS, Lee WL, Shu CM. Hazard evaluation for redox system of cumene hydroperoxide mixed with inorganic alkaline solutions. J Therm Anal Calorim. 2009;95(2):541–5.
Wang YW, Duh YS, Shu CM. Thermal runaway hazards of tert-butyl hydroperoxide by calorimetric studies. J Therm Anal Calorim. 2009;95(2):553–7.
Wang YW, Duh YS, Shu CM. Characterization of the self-reactive decomposition of tert-butyl hydroperoxide in three different diluents. Process Safety Progress. 2007;26(4):299–303.
Barton JA, Nolan PF. Incidents in the chemical industry due to thermal-runaway chemical reactions, hazards X. Process safety in fine and specialty chemical plants. Int Chem E Symp Ser. 1989;115:3–18.
Wu SH, Chi JH, Huang CC, Lin NK, Peng JJ, Shu CM. Thermal hazard analyses and incompatible reaction evaluation of hydrogen peroxide by DSC. J Therm Anal Calorim. 2010;102:563–8.
Kao CS, Hu KH. Acrylic reactor runaway and explosion accident analysis. J Loss Prev Process Ind. 2002;15:213–22.
Cheng SY, Tseng JM, Lin SY, Gupta JP, Shu CM. Runaway reaction on tert-butyl peroxybenzoate. J Therm Anal Calorim. 2008;93(1):121–6.
Laine DF, Roske CW, Cheng IF. Electrochemical detection of triacetone triperoxide employing the electrocatalytic reaction of iron (II/III)-ethylenediaminetetraacetate and hydrogen peroxide. Anal Chim Acta. 2008;608:56–60.
Singh S. Sensors—an effective approach for the detection of explosives. J Hazard Mater. 2007;144:15–28.
Kao CS, Duh YS, Chen JH, Yu SW. An index-based method for assessing exothermic runaway risk. Process Safety Progress. 2002;21(4):294–304.
Wikipedia. (2009). http://en.wikipedia.org/.
Tallman RL, Margrave JL, Bailey SW. The crystal structure of sodium peroxide. J Am Chem Soc. 1957;79:2979–80.
Wu SH. Process safety application and loss prevention of cumene hydroperoxide. PhD dissertation, National Yunlin University of Science and Technology (NYUST), Douliou, 2010.
Lin ML. Thermal hazard evaluation of tert-butyl peroxide (TBPO) using calorimetric approaches. PhD dissertation, National Yunlin University of Science and Technology (NYUST), Douliou, 2010.
Lin ML, Shen SJ, Wu SH, Shu CM. Thermal explosion simulation of hydrogen peroxide in three types of vessels by explosion models. Res J Chem Environ. 2010;14(2):88–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, SH., Chou, HC., Pan, RN. et al. Thermal hazard analyses of organic peroxides and inorganic peroxides by calorimetric approaches. J Therm Anal Calorim 109, 355–364 (2012). https://doi.org/10.1007/s10973-011-1749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1749-5