Abstract
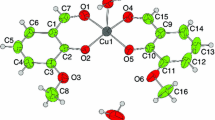
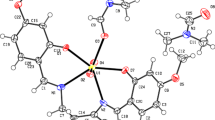
The coordination complex of Cu (II) with the Schiff base derived from 4-chloroaniline with salicylaldehyde have been synthesized and characterized by micro analytical data; FT–IR, UV–Vis, FAB-mass and thermal analysis studies. Thermal data show degradation of complexes. We carried out thermal analysis at three different heating rates viz. 5, 10 and 20 °C per min. The activation thermodynamic parameters, such as activation energy (E*), entropy of activation (ΔS*), enthalpy of activation (ΔH*) and Gibbs free energy (ΔG*) have been calculated with the help of TG, DTA and DTG curves using Coats–Redfern method. The stoichiometry of the complexes are in 1:2 (M:L) molar ratio. Synthesized complex has been tested for their reactivity and substitution behaviour.




Similar content being viewed by others
References
Brown ME, Dollimore D, Galwey AK. Reactions in the solid state, comprehensive chemical kinetics. Elsevier: Amsterdam; 1980. p. 340.
Brown ME. Introduction to thermal analysis. Techniques and applications. 2nd ed. London: Kluwer Academic Publishers; 2001. p. 264.
Al-Maydama H, El-Shekeil A, Khalid MA, Al-Karbouly A. Thermal degradation behavior of some polydithiooxamide. Ecl Quim. 2006;31(1):45–52.
Ahmed AA, BenGuzzi SA, Agumati S. Synthesis and characterization of binuclear Cu(II) complexes of some Schiff base ligand. Garyounis Univ Press J Sci Appl. 2009;3(1):112–20.
Kamruddin M, Ajikumar PK, Dash S, Tyagi AK, Raj B. Thermogravimetry-evolved gas analysis mass spectrometry system for materials research. Bull Mater Sci. 2003;22(4):449–60.
Isola M, Liuzzo V, Marchetti F. Synthesis and characterization of the dihydrosalpren ligand (H2L1) and of its trinuclear Ni(II)complex. Inorg Chem Commun. 2009;12:608–10.
Yu Z, He M, Sun P, Zhang W, Chang L. Crystal structure of trinuclear nickel(II) complex with Schiff base ligand N, N0-bis(salicylidene)-1, 3-diiminopropane. J Chem Crystallogr. 2009;39:885–9.
You Z-L. Syntheses and crystal structure of two novel linear trinuclear Schiff base nickel(II) and cadmium(II) complexes. Z Anorg Allg Chem. 2006;632:664–8.
Arslan F, Odabazoglu M, Olmez H, Buyukgungor O. Synthesis, crystal structure, spectral and thermal characterization of bis (o-vanillinato)-triethylenglycoldiimine copper(II) and bis[(R)-(-)-hydroxymethylpropylimine-o-vanillinato] copper(II). Polyhedron. 2009;28:2943–8.
Thakurta S, Rizzoli C, Butcher RJ, Gomez-Garcia CJ, Garribba E, Mitra S. Sterically controlled nuclearity in new copper(II) complexes with di-compartmental ligands: formation of antiferromagnetically coupled angular trimer and mononuclear inclusion complex. Inorg Chim Acta. 2010;363:1395–403.
Khalaji AD, Stoeckli-Evans H. Tetranuclear azido-bridged copper(II) complex [Cu4(lsalpn)2(l1, 1–N3)2(N3)2(H2O)2]: synthesis, characterization and crystal structure. Polyhedron. 2009;28:3769–73.
Khalaji AD, Hadadzadeh H, Fejfarova K, Dusek M. Metaldependent assembly of a tetranuclear copper(II) complex versus a 1D chain coordination polymer of cobalt(III) complex with N2O2-chelating Schiff-base ligand: synthesis, characterization and crystal structures. Polyhedron. 2010;29:807–12.
Feltham HLC, Clerac R, Brooker S. Hexa-, hepta- and dodecanuclear nickel(II) complexes of three Schiff-base ligands derived from 1, 4-diformyl-2, 3-dihydroxybenzene. Dalton Trans. 2009;29:65–73.
Dong WK, Chen X, Sun YX, Yang YH, Zhao L, Xu L, Yu TZ. Synthesis, structure and spectroscopic properties of two new trinuclear nickel(II) clusters possessing solvent effect. Spectrochim Acta. 2009;A74:719–25.
Nayak M, Sarkar S, Lemoine P, Sasmal S, Koner R, Sparkes HA, Howard JAK, Mohanta S. Suramolecular dimmers of copper(II) complexes resulting from designed host-guest interactions. Eur J Inorg Chem. 2010;5:744–52.
Biswas M, Pilet G, Tercero J, El Fallah MS, Mitra S. Synthesis, crystal structure and magnetic properties of a new tetranuclear Cu(II) Schiff base compound. Inorg Chim Acta. 2009;362:2915–20.
Koner S, Saha S, Okamoto K-I, Tuchagues J-P. A novel tetranuclear copper(II) complex with alternating l1, 1-azido and phenoxo bridges: synthesis, structure and magnetic properties of [Cu4(l-salen)2(l1, 1–N3)2(N3)2]. Inorg Chem. 2003;42:4668–72.
Masoud MS, Refaat LS. Synthesis and characterization of some nickel(II) Schiff bases complexes. Trans Met Chem. 1982;7:315–8.
Abdel-Gaber AM, Masoud MS, Khalil EA, Shehata EE. Electrochemical study on the effect of Schiff base and its cobalt complex on the acid corrosion of steel. Corros Sci. 2009;51:3021–4.
Oz S, Kunduraci M, Kurtaran R, Ergun U, Arici C, Akay MA, Atakol O, Emregul KC, Ulku D. Thermal decomposition of linear tetranuclear copper(II) complexes including l-azido bridges. J Therm Anal Calorim. 2010;101:221–7.
Abdel-Fattah HM, El-Ansary AL, Abdel-Kader NS. Thermal and spectral studies on complexes derived from tetradentate Schiff bases. J Therm Anal Calorim. 2009;96:961–9.
Kriza A, Dianu ML, Andronescu C, Rogozea E, Musuc AM. Synthesis, spectral and thermal studies of new copper(II) complexes with 1, 2-di(imino-2-aminomethylpyridil)ethane. J Therm Anal Calorim. 2010;100:929–35.
Dogan F, Ulusoy M, Ozturk OF, Kaya I, Salih B. Synthesis, characterization and thermal study of some tetradentate Schiff base transition metal complexes. J Therm Anal Calorim. 2009;98:785–92.
Zeybek B, Ates BM, Ercan F, Aksu ML, Kilic E, Atakol O. The effect of ligand basicity on the thermal stability of heterodinuclear NiII–ZnII complexes. J Therm Anal Calorim. 2009;98:377–85.
Avsar G, Altinel H, Yilmaz MK, Guzel B. Synthesis, characterization and thermal decomposition of fluorinated salicylaldehyde Schiff-base derivatives (salen) and their complexes with copper(II). J Therm Anal Calorim. 2010;101:199–203.
Durmus S, Ergun U, Jaud JC, Emregul KC, Fuess H, Atakol O. Thermal decomposition of some linear trinuclear Schiff base complexes with acetate bridges. J Therm Anal Calorim. 2006;86:337–46.
Aksu M, Durmus S, Sari M, Emregul KC, Svoboda I, Fuess H, Atakol O. Investigation on the thermal decomposition some heterodinuclear NiII–MII complexes prepared from ONNO type reduced Schiff base compounds (MII=ZnII, CdII). J Therm Anal Calorim. 2007;90:541–7.
Oz S, Kurtaran R, Arici C, Ergun U, Kaya FND, Emregul KC, Atakil O, Ulku D. Two non-linear azide containing heteronuclear complexes: crystal structure and thermal decomposition. J Therm Anal Calorim. 2010;99:363–8.
Khalaji AD, Rad SM, Grivani G, Das D. Nickel(II) and copper(II) complexes with an asymmetric bidentate Schiff-base ligand derived from furfurylamine :Synthesis, spectral, XRD, and thermal studies. J Therm Anal Calorim. 2011;103:747–51.
Coats AV, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Acknowledgements
The authors are thankful to Mr. Harshil Shah, Mettler Toledo India Private Limited, Powai Mumbai India for Thermal analysis. The financial assistance from U.G.C. New Delhi to SS is also thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shukla, S., Mishra, A.P. Non-isothermal degradation-based solid state kinetics study of copper (II) Schiff base complex, at different heating rates. J Therm Anal Calorim 107, 111–117 (2012). https://doi.org/10.1007/s10973-011-1616-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1616-4