Abstract
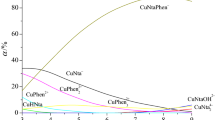
The mixed-ligand complex formation in the systems Cd2 + Edta4–(CH2) n (NH2)2, n = 2 (En), 6 (L) has been NMR and calorimetrically studied in aqueous solution at 298.15 K and the ionic strength of I = 0.5 (KNO3). The thermodynamic parameters of formation of the CdEdtaL2−, CdEdtaHL−, (CdEdta)2L4−, and (CdEdta)2En4− complexes have been determined. The most probable coordination mode for the complexone and the diamine ligand in the mixed-ligand complexes was discussed.



Similar content being viewed by others
References
Wang XF, Wang YF, Wang J, Zhang ZhH, Gao J, Liu B, Jiang YCh, Zhang XD. Syntheses and structures of seven-coordinate K3[Cd(Dtpa)], K2[Cd(H2O)4][Cd(Edta)(H2O)]2·2H2O, and Na2[Cd(H2O)4][Cd(Edta)(H2O)]2·2H2O complexes. Rus J Coord Chem. 2008;34:563–70.
Kozlovskii EV. Doctoral (Chem.) Dissertation, Ivanovo State University, Ivanovo; 1995.
Pyreu DF, Titova ES, Kozlovskii EV. Thermodynamics of mixed-ligand complex formation of mercury(II) ethylenediaminetetraacetate with ethylenediamine and hexamethylenediamine in an aqueous solution. Rus J Inorg Chem. 2008;53:334–6.
Pyreu DF, Kozlovskii EV. Thermodynamics of mixed-ligand complex formation of copper(II) ethylenediaminetetraacetate with hexamethylenediamine in an aqueous solution. J Therm Anal Calorim. 2010;100:355–60.
Mah V, Jalilehvand F. Mercury (II) complex formation with glutathione in alkaline aqueous solution. J Biol Inorg Chem. 2008;13:541–53.
Mah V, Jalilehvand F. Cadmium(II) complex formation with glutathione. J Biol Inorg Chem. 2010;15:441–58.
Borodin VA, Vasiliev VP, Kozlovskii EV. Mathematical problems in chemical thermodynamics. Novosibirsk: Nauka; 1985.
Vasiliev VP. Thermodynamic properties of electrolyte solutions. Moscow: Vysschaya Shkola; 1982.
Borodin VA. Cand Sci (Chem.). Dissertation, Ivanovo Institute of Chemical Technology, Ivanovo; 1983.
Kozlovskii EV, Fridman AYa. Structural and thermodynamic features of addition of mono- and bidentate ligands to the nickel, copper and zinc ethylenediaminetetraacetates in an aqueous solution. Rus J Inorg Chem. 1991;36:1500–2.
Day RJ, Reilley CN. Nuclear magnetic resonance studies of metal aminopolycarboxylate complexes. Lability of individual metal ligand bonds in (ethylenedinitrilo)tetraacetate complexes. Anal Chem. 1964;36:1073–6.
Jencen CF, Deshmukh S, Jakobsen HJ, Inners RR, Ellis PD. Cadmium-113 nuclear magnetic resonance studies of cadmium-ethylenediaminetetraacetic acid complexes. J Am Chem Soc. 1981;103:3659–66.
Rabenstein DL, Blakney G, Fuhr BJ. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. XIII. The lability of the metal-nitrogen bonds in the cadmium, zinc, and mercury complexes of selected polyaminocarboxylic acids. Can J Chem. 1975;53:787–91.
Acknowledgements
This study was supported by the Ministry of Education and Science of the Russian Federation, Project No. P1360 (Scientific and scientifically pedagogical personnel of Russia during 2009–2013) and the President of the Russian Federation, Grant No. MK-1625.2009.3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dmitrii, P., Eugenii, K., Matvei, G. et al. Thermodynamic and NMR studies of mixed-ligand complex formation of cadmium ethylenediaminetetraacetate with diamines in an aqueous solution. J Therm Anal Calorim 103, 1073–1077 (2011). https://doi.org/10.1007/s10973-010-1138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1138-5