Abstract
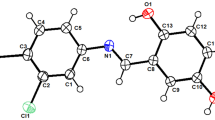
Indomethacin crystallizes from solutions in tetrahydrofuran as a solvate exhibiting the mole ratio 1 indomethacin:2 tetrahydrofuran. Upon heating, desolvation into indomethacin phase I occurs through partial amorphization and transitory formation of a phase, which is different from the crystallographically known polymorphs. The X-ray powder diffraction pattern of the solvate was tentatively indexed on a triclinic lattice (a = 31.454(5) Å, b = 17.883(3) Å, c = 10.551(2) Å, α = 70.55(2)°, β = 105.31(2)°, γ = 136.70(1)°). Assuming Z = 6 (1 indomethacin + 2 tetrahydrofuran) formula units per unit cell, the solvate’s specific volume is similar to the value calculated using additivity.








Similar content being viewed by others
References
Yamamoto H. 1-Acyl-indoles. II. A new syntheses of 1-(ion-chlorobenzoyl)-5-methoxy-3-indolylacetic acid and its polymorphism. Chem Pharm Bull (Tokyo). 1968;16:17–9.
Borka L. Polymorphism of indomethacine. New modifications, their melting behavior, and solubility. Acta Pharm Suec. 1974;11:295–303.
Crowley KJ, Zografi G. Cryogenic grinding of indomethacin polymorphs and solvates: assessment of amorphous phase formation and amorphous phase physical stability. J Pharm Sci. 2002;91:492–507.
Spychala S, Butkiewicz K, Pakula R, Pichnej L. Polymorphism of indomethacin. Part II. Identification and rapid determination of polymorphic forms of indomethacin by IR spectrometry. Pol J Pharmacol Pharm. 1977;29:157–60.
Kaneniwa N, Otsuka M, Hayashi T. Physicochemical characterization of indomethacin polymorphs and the transformation kinetics in ethanol. Chem Pharm Bull. 1985;33:3447–55.
Yoshioka M, Hancock BC, Zografi G. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. J Pharm Sci. 1994;83:1700–5.
Legendre B, Feutelais Y. Polymorphic and thermodynamic study of indomethacin. J Therm Anal Calorim. 2004;76:255–64.
Hamdi N, Feutelais Y, Yagoubi N, de Girolamo D, Legendre B. Solvates of indomethacin. J Therm Anal Calorim. 2004;76:985–1001.
Pan X, Julian T, Augsburger L. Quantitative measurement of indomethacin crystallinity in indomethacin-silica gel binary system using differential scanning calorimetry and X-ray powder diffractometry. AAPS PharmSciTech. 2006;7:E11.
Kistenmacher TJ, Marsh RE. Crystal and molecular structure of an antiinflammatory agent, indomethacin, 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid. J Am Chem Soc. 1972;94:1340–5.
Galdecki Z, Glowka ML. Crystal and molecular structure of the gamma-form of 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid. A comparison of results based on photographic data with previous results obtained by means of a single crystal diffractometer. Rocz Chem. 1976;50:1139–48.
Cox PJ, Manson PL. γ-Indomethacin at 120 K. Acta Crystallogr Sect E Struct Rep Online. 2003;E59:o986–8.
Chen X, Morris KR, Griesser UJ, Byrn SR, Stowell JG. Reactivity differences of indomethacin solid forms with ammonia gas. J Am Chem Soc. 2002;124:15012–9.
Cox PJ, Manson PL. Indomethacin tert-butanol solvate at 120 K. Acta Crystallogr Sect E Struct Rep Online. 2003;E59:o1189–91.
Slavin PA, Sheen DB, Shepherd EEA, Sherwood JN, Feeder N, Docherty R, et al. Morphological evaluation of the gamma-polymorph of indomethacin. J Cryst Growth. 2002;237–239:300–5.
Rodriguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B (Amsterdam). 1993;192:55–69.
Rodriguez-Carvajal J. Commission on powder diffraction (IUCr). Newsletter. 2001;26:12.
Boultif A, Louer D. Powder pattern indexing with the dichotomy method. J Appl Crystallogr. 2004;37:724–31.
Luger P, Buschmann J. Twist conformation of tetrahydrofuran in the crystal form. Angew Chem Int Ed. 1983;22:410–1.
Steininger R, Bilgram JH, Gramlich V, Petter W. Crystal growth, crystal optics, and crystal structure of the phase IV of tertiary-butyl alcohol. Z Kristallogr. 1989;187:1–13.
McGregor PA, Allan DR, Parsons S, Clark SJ. Hexamer formation in tertiary butyl alcohol (2-methyl-2-propanol, C4H10O). Acta Crystallogr Sect B Struct Sci. 2006;B62:599–605.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nicolaï, B., Céolin, R. & Rietveld, I.B. Polymorphism and solvation of indomethacin. J Therm Anal Calorim 102, 211–216 (2010). https://doi.org/10.1007/s10973-009-0412-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0412-x