Abstract
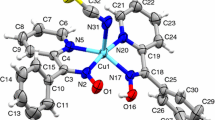
In the first instance, mononuclear Cu(II) complexes are prepared with bis-N,N′(salicylidene)-1.3-propanediamine and derivatives. After that, these mononuclear complexes are combined with μ-bridges, by the help of azide ions, to obtain the tetranuclear complexes. Prepared complexes are characterised using IR spectroscopy, elemental analysis, and X-Ray techniques. In addition, the complexes are further analysed via TG and DSC. Molecular models of two of the nine prepared complexes are determined using X-Ray diffraction methods. The two terminal copper ions are observed to be in square pyramide coordination sphere between two oxygens of the organic ligand, two iminic nitrogens and an oxygen of the solvent while the other two cupper ions are observed to be in square pyramide coordination sphere between the fenolic oxygens of the organic ligand and the nitrogen donors of the three azide ions. It is found that the fenolic oxygens form μ-bridge and two azide ions are monodentate coordinated. In the TG analyses, the complexes are observed to decompose in a highly exothermic manner at about 200 °C. This thermal reaction is partially similar to that of explosive molecules and the data from DSC proved that the liberated heat is at explosive material levels.





Similar content being viewed by others
References
Charlot MF, Kahn O, Chaillet M, Larrieu C. Interaction between copper(II) ions through the azido bridge: concept of spin polarization and ab initio calculations on model systems. J Am Chem Soc. 1986;108:2574–81.
Cortes R, de Larramendi JIR, Lezama L, Rojo T, Urtiaga K, Arriortua MI. Synthesis, structural, spectroscopic and magnetic studies of two azido and thiocyanato nickel(II) dinuclear complexes with ferromagnetic interactions. JCS Dalton Trans. 1992; 2723–8.
Julve M, Verdaguer M, de Munno D, Real JA, Bruno G. Synthesis, crystal structure, and magnetic properties of (μ-bipyrimidine)(cyanato)copper(II) and -(thiocyanato)copper(II) complexes. Inorg Chem. 1993;32:795–802.
Ribas J, Escuer A, Monfort M, Vicente R, Cortes R, Lezama L, et al. Polynuclear NiII and MnII azido bridging complexes. Structural trends and magnetic behavior. Coord Chem Rev. 1999;193–195:1027–68.
Goher MAS, Escuer A, Mautner FA, Al-Salem NA. Synthesis, spectral, magnetic and crystal structural characterization of two new copper(II) azido complexes: catena-[μ(N3)Cu(pyridine)3] n (PF6) n and dimeric [Cu(4-ethylpyridine)(N3)2]2. Polyhedron. 2001;20:2971–7.
Gao EQ, Bai SQ, Wang CF, Yue YF, Yan CH. Structural and magnetic properties of three one-dimensional azido-bridged copper(II) and manganese(II) coordination polymers. Inorg Chem. 2003;42:8456–64.
Cabort A, Therrien B, Bernauer K, Süss-Fink G. Copper(II) azido complexes containing trinitrogen ligands: [Cu(η3-L)(N3)]2[Cu2Cl2(N3)4] [L=2,6-bis(3,4-dihydro-2H-pyrrol-5-yl)pyridine], a tridimensional network of cationic and anionic copper complexes. Inorg Chim Acta. 2003;349:78–84.
Deoghoria S, Sain S, Sola M, Wong WJ, Christov G, Bera SK, et al. Synthesis, crystal structure and magnetic properties of a new ferromagnetic nickel(II) dimer derived from a hexadentate Schiff base ligand. Polyhedron. 2003;22:257–62.
Jia HP, Li W, Ju ZF, Zhang J. Synthesis, structure, and magnetic properties of a novel mixed-bridged heterometal tetranuclear complex [Mn2Ni2(MeOSalen)2(μ1,1-N3)2(N3)2]. Inorg Chem Commun. 2007;10:397–400.
Liu CM, Zhang DQ, Zhu DB. A copper(II) coordination polymer with alternating double EO-azido bridges and mixed EO-azido/alkoxo double bridges. Inorg Chim Acta. 2009;362:1383–6.
Fukuhara C, Tsuneyoshi K, Matsumoto N, Kida S, Mikuriya M, Mori M. Synthesis and characterization of trinuclear Schiff-base complexes containing sulphur dioxide or hydrogen-sulphite ions as bridging groups. Crystal structure of [Zn{(μ-CH3CO2)(salpd-μ-O,O′)Cu}2][salpd = propane-1,3-diylbis(salicylideneiminate)]. JCS Dalton Trans 1990:3473–9.
Uhlenbrock S, Wegner R, Krebs B. Syntheses and characterization of novel tri- and hexa-nuclear zinc complexes with biomimetic chelate ligands. JCS Dalton Trans 1996: 3731–6.
Mikuriya M, Tsuru N, Ikemi S, Ikenoue S. High nuclearity in a zinc(II) complex with 1,3-bis(salicylamino)-2-propanol. Chem Lett 1998;9:879–80.
Mikuriya M, Ikenoue S, Nukada R, Lim JW. Synthesis and structural characterization of tetranuclear zinc(II) complexes with a linear array. Bull Chem Soc Jpn. 2001;74:101–2.
Atakol O, Durmuş S, Durmuş Z, Arıcı C, Çiçek B. Investigations, on some heterotrinuclear complexes of nickel(II) and copper(II). Synth React Inorg Met Org Chem. 2001;31:1689–704.
Reglinski J, Taylor MK, Kennedy AR. Hydrogenated Schiff base ligands: towards the controlled organisation of open metal frameworks. Inorg Chem Commun. 2006;9:736–9.
Shi DH, You ZL, Xu C, Zhang Q, Zhu HL. Synthesis, crystal structure and urease inhibitory activities of Schiff base metal complexes. Inorg Chem Commun. 2007;10:404–6.
Drew MGB, Prasad RN, Sharma RP. Structures of (N,N′-trimethylenedisalicylideneaminato) nickel(II) (1) and (N,N′-trimethylenedisalicylideneaminato) copper(II) (2). Acta Crystallogr C. 1985;C41:1755–8.
Arıcı C, Ercan F, Kurtaran R, Atakol O. [N,N′-bis(salicylidene)-2,2-dimethyl-1,3-propanediaminato] nickel(II) and [N,N′-bis(salicylidene)-2,2-dimethyl-1,3-propanediaminato] copper(II). Acta Crystallogr C. 2001;C57:812–4.
Yua HH, Lo JM, Chen BH, Lu TH. [N,N′-bis(salicylidene)-1,4-diiminobutane] copper(II). Acta Crystallogr C. 1997;C53:1012–3.
Butcher RJ, Sinn E. Relation between magnetic, spectroscopic, and structural properties of bis[chloro(N-isopropyl-2-hydroxybenzylidene)aminato-μ-O-copper(II)] and bis(N-isopropyl-2-hydroxybenzylideneaminato)copper(II). Inorg Chem. 1976;15:1604–8.
Kurtaran R, Emregül KC, Arıcı C, Ercan F, Catalano VC, Atakol O. Synthesis and crystal structure of linear chain homotetranuclear complexes with N3 −. Synth React Inorg Met Org Chem. 2003;33:281–96.
Koner S, Saha S, Okamato KI, Tuchagues JP. A novel tetranuclear copper(II) complex with alternating μ1,1-azido and phenoxo bridges: synthesis, structure, and magnetic properties of [Cu4(μ-salen)2(μ1,1-N3)2(N3)2]. Inorg Chem. 2003;42:4668–72.
Reddy KR, Rajasekharan MV, Tuchagues JP. Synthesis, structure, and magnetic properties of Mn(salpn)N3, a helical polymer, and Fe(salpn)N3, a ferromagnetically coupled dimer (salpnH2 = N,N′-bis(salicylidene)-1,3-diaminopropane). Inorg Chem. 1998;37:5978–82.
Enraf Nonius:Cad-4 Express Version 1.1. Delft, The Netherlands; 1993.
Sheldrick GM. SHELXS97 and SHEXL97. Program for crystal structure solution and refinement. University of Gottingen, Germany; 1997.
Farrugia LJ. WinGX. Program for crystallography package. J Appl Crystallogr. 1999;32:837.
Spek AL. PLATON. Program for crystal molecular drawing. The Netherlands: University of Ultrech; 2000.
Banerjee S, Ray A, Sen S, Mitra S, Hughes DL, Butcher RJ, et al. Pseudohalide-induced structural variations in hydrazone-based metal complexes: syntheses, electrochemical studies and structural aspects. Inorg Chim Acta. 2008;361:2692–700.
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. JCS Dalton Trans 1984:1349–56.
Wang QL, Yang C, Qi L, Liao DZ, Yang GM, Ren HX. A trinuclear nickel(II) complex with dissimilar bridges: synthesis, crystal structure, spectroscopy and magnetism. J Mol Struct. 2008;892:88–92.
Khalaji AD, Amirnasr M, Triki S. New coordination polymer based on salpn Schiff base and azide bridging ligands: synthesis and structural characterization of {Na[CoIII(μ-salpn)(μ1,1-N3)2]} n (H2salpn = N,N′-bis(salicylidene)-1,3-diaminopropane). Inorg Chim Acta. 2009;362:587–90.
Liu Z, Zhang T, Zhang J, Wang S. Studies on three-dimensional coordination polymer [Cd2(N2H4)2(N3)4] n : crystal structure, thermal decomposition mechanism and explosive properties. J Hazard Mater. 2008;154:832–8.
Demeshko S, Leibeling G, Marringgele W, Meyer F, Mennerich C, Klauss HH, et al. Structural variety and magnetic properties of tetranuclear nickel(II) complexes with a central μ4-azide. Inorg Chem. 2005;44:519–28.
Dinçer Kaya FN, Svoboda I, Atakol O, Ergun Ü, Kenar A, Sarı M, et al. Nickel(II) complexes prepared from NNN type ligands and pseudohalogens—synthesis, structure and thermal decomposition. J Therm Anal Calorim. 2008;92:617–24.
Soliman AA, Linert W. Investigations on new transition metal chelates of the 3-methoxy-salicylidene-2-aminothiophenol Schiff base. Thermochim Acta. 1999;338:67–75.
El-Said AI. Studies on some nickel(II) and cobalt(II) mixed ligand complexes of arylsalicyl-aldimine and other ligands. J Therm Anal Calorim. 2002;68:917–29.
Aranha PE, Souza JM, Romero S, Ramos LA, dos Santos MP, Dockal ER, et al. Thermal behavior of vanadyl complexes with Schiff bases derived from trans-N,N′-bis(salicylidene)-1,2-cyclohexadiamine (t-Salcn). Thermochim Acta. 2007;453:9–13.
Acknowledgements
The authors wish to acknowledge the financial support of the Ankara University Research Fund (Project no. 07B4240001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öz, S., Kunduracı, M., Kurtaran, R. et al. Thermal decomposition of linear tetranuclear copper(II) complexes including μ-azido bridges. J Therm Anal Calorim 101, 221–227 (2010). https://doi.org/10.1007/s10973-009-0394-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0394-8