Abstract
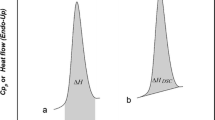
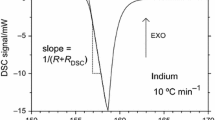
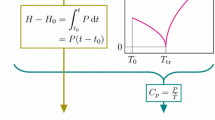
High-temperature differential scanning calorimetry was used to investigate the thermodynamic parameters of the γ–β and β–α transitions in calcium pyrophosphate (Ca2P2O7). The measured enthalpy of transition compared well with previous results when higher heating rates (≥20 K min−1) were used. Recommendations for optimal use of HTDSC in high-temperature phase transition measurements are presented.





Similar content being viewed by others
References
Naumann R. Heat capacity measurement via scanning method at high temperatures. J Therm Anal. 1998;53:659–69.
Emmerich W-D, Janoschek J, Kaisersberger E. Recent advantages in thermal analysis instrumentation. J Therm Anal. 1989;35:1067–72.
Blumm J, Kaisersberger E. Accurate measurement of transformation energetics and specific heat by DSC in the high-temperature region. J Therm Anal Calorim. 2001;64:385–91.
Jacob S, Schlesinger ME. A study of accuracy and reproducibility of high temperature differential scanning calorimetry. In: Kongoli F, Reddy RG, editors. Advanced processing of metals and materials, vol. 1: thermo and physicochemical principles: nonferrous high temperature processing. Warrendale, PA: TMS-AIME; 2006. p. 209–18.
Jacob S, Schlesinger ME. Evaluation of the accuracy and reproducibility of a high temperature differential scanning calorimeter by heat capacity measurements. Am Lab. 2006;38:13–5.
Hill WL, Faust GT, Reynolds DS. The binary system P2O5–2CaO∙P2O5. Am J Sci. 1944;242:457–77, 542–62.
Egan EP, Wakefield ZT. Thermodynamic properties of calcium pyrophosphate, 10 to 1700 K. J Am Chem Soc. 1957;79:558–61.
Jungowska W, Znamierowska T. LaPO4–Ca2P2O7 system. Prace Naukowe Akademii Ekonomicznej imienia Oskara Langego we Wroclawiu. 1994;67:358–60.
Monma H. Behavior of the β ↔ α transformation in calcium pyrophosphate. Gypsum Lime. 1990;225:77–81.
Wikholm NW, Beebe RA, Kittelberger JS. Kinetics of the conversion of monetite to calcium pyrophosphate. J Phys Chem. 1975;79:853–6.
Shaw TL, Carrol JC. Application of baseline correction techniques to the “ratio method” of DSC specific heat determination. Int J Thermophys. 1998;19:1671–80.
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant DMR-0404605. The assistance of Darlene Ramsay and Eric Bohannan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacob, S., Schmitt, M.L. & Schlesinger, M.E. Use of high-temperature differential scanning calorimetry to investigate the β–α transition in calcium pyrophosphate. J Therm Anal Calorim 100, 897–900 (2010). https://doi.org/10.1007/s10973-009-0373-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0373-0