Abstract
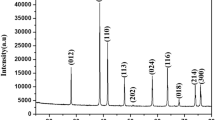
This article presents a study on obtaining Ni, Zn ferrite starting from Fe(III), Ni (II), Zn (II) nitrates and some polyols: 1,2-propane diol, 1,3-propane diol and glycerol. While heating, a redox reaction takes place between nitrate anion and polyol, with formation of carboxylate type precursors. The obtained precursors have been investigated by thermal analysis, FT-IR spectrometry and atomic absorption spectroscopy. The thermal decomposition of the synthesized precursors up to 350 °C leads to the formation of Ni, Zn ferrite as unique phase, evidenced by XRD. The average diameter of the ferrite crystallites, estimated from XRD data, takes values within the range 20–50 nm, depending on the annealing temperature. Transmission Electron Microscopy has evidenced the obtaining of spherical, agglomerated nanoparticles. The magnetic properties of the synthesized samples, measured in cvasistatic magnetic field (50 Hz) are characteristic for the Ni, Zn ferrite nanoparticles, with narrow hysteresis cycle and values of the saturation magnetization <70 emu/g.









Similar content being viewed by others

References
Costa ACFM, Tortella E, Morelli MR, Kiminami RHGA. Synthesis, microstructure and magnetic properties of Ni–Zn ferrites. J Magn Magn Mater. 2003;256:174–82.
Eftekhari A, Molaei F, Arami H. Flower-like bundles of ZnO nanosheets as an intermediate between hollow nanosphere and nanoparticles. Mater Sci Eng: A. 2006;437:446–50.
Illy-Cherrey S, Tillement O, Dubois JM, Massicot F, Fort Y, Ghanbaja J, et al. Synthesis and characterization of nano-sized nickel(II), copper(I) and zinc(II) oxide nanoparticles. Mater Sci Eng: A. 2002;338:70–5.
More A, Verenkar VMS, Mojumdar SC. Nickel ferrite nanoparticles synthesis from novel fumarato-hydrazinate precursor. J Therm Anal Calorim. 2008;94:63–7.
Randhawa BS, Kaur M. A comparative study on the thermal decomposition of some transition metal maleates and fumarates. J Therm Anal Calorim. 2007;89:251–5.
Carp O, Segal E, Brezeanu M, Barjega R, Staniac N. Nonconventional methods for obtaining hexaferrites. J Therm Anal Calorim. 1997;50:125–35.
Bakalova A. Thermal and spectroscopic investigation of new binuclear Pt(II) complexes with carboxylic acids. J Therm Anal Calorim. 2009;96:593–7.
Logvinenko VA. Thermoanalytical approach to the study of the kinetic and thermodynamic stability of coordination compounds and clathrates. J Therm Anal Calorim. 1990;36:1973–80.
Stefanescu M, Sasca V, Birzescu M. Thermal behaviour of the homopolynuclear glyoxylate complex combinations with Cu(II) and Cr(III). J Therm Anal Calorim. 2003;72:515–24.
Kingsley JJ, Patil KC. A novel combustion process for the synthesis of fine particle α-alumina and related oxide materials. Mater Lett. 1988;6:427–32.
Deka S, Joy PA. Characterization of nanosized NiZn ferrite powders synthesized by an autocombustion method. Mater Chem Phys. 2006;100:98–101.
Hwang C-C, Tsai JS, Huang T-H. Combustion synthesis of Ni–Zn ferrite by using glycine and metal nitrates—investigations of precursor homogeneity, product reproducibility, and reaction mechanism. Mater Chem Phys. 2005;93:330–6.
Mangalaraja RV, Ananthakumar S, Manohar P, Gnanam FD. Initial permeability studies of Ni–Zn ferrites prepared by flash combustion technique. Mater Sci Eng: A. 2003;355:320–4.
Sorescu M, Diamandescu L, Peelamedu R, Roy R, Yadoji P. Structural and magnetic properties of NiZn ferrites prepared by microwave sintering. J Magn Magn Mater. 2004;279:195–201.
Anil Kumar PS, Shrotri JJ, Kulkarni SD. Low temperature synthesis of Ni0.8Zn0.2Fe2O4 powder and its characterization. Mater Lett. 1996;27:293–6.
Wu KH, Chang YC, Chang TC. Effects of SiO2 content and solution pH in raw materials on Ni–Zn ferrite magnetic properties. J Magn Magn Mater. 2004;283:380–4.
Abdeen AM. Electric conduction in Ni–Zn ferrites. J Magn Magn Mater. 1998;185:199–206.
Arshak KI, Ajina A, Egan D. Development of screen-printed polymer thick film planner transformer using Mn–Zn ferrite as core material. Microelectron J. 2001;32:113–6.
Bhise BV, Dongare MB, Patil SA. X-ray infrared and magnetization studies on Mn substituted Ni-Zn ferrites. J Mater Sci Lett. 1991;10:922–4.
Caizer C, Ştefănescu M. Magnetic characterization of nanocrystalline Ni–Zn ferrite powder prepared by the glyoxylate precursor method. J Phys D. 2002;35:3035–40.
Niculescu M, Vaszilcsin N, Bîrzescu M, Budrugeac P, Segal E. Thermal and structural investigation of the reaction between 1,2-propanediol and Ni(NO3)2.6H2O. J Therm Anal Calorim. 2001;63:181–5.
McMorn P, Roberts G, Hutchings GJ. Oxidation of glycerol with hydrogen peroxide using silicalite and aluminophosphate catalysts. Catal Lett. 1999;63:193–7.
Nakamoto K. Infrared spectra of inorganic and coordination compounds. New York: Wiley; 1970. pp. 201, 199, 159, 217.
Prasad R, Sulaxna, Kumar A. Kinetics of thermal decomposition of iron(iii) dicarboxylate complexes. J Therm Anal Calorim. 2005;81:441–50.
Dranca I, Lupascu T, Sofransky V, Popa V, Vass M. Thermoanalytical study of some salts of 3d metals with d-tartaric acid. J Therm Anal Calorim. 1996;46:1403–12.
Zelenak V, Vargova Z, Gyoryova K. Correlation of infrared spectra of zinc(II) carboxylates with their structures. Spectrochim Acta A. 2007;66:262–72.
Stefanescu M, Stefanescu O, Stoia M, Lazau C. Thermal decomposition of some metalorganic precursors: Fe2O3 nanoparticles. J Therm Anal Calorim. 2007;88:27–32.
Suryanarayana C, Grant Norton M. X-ray diffraction. A practical approach. New York, London: Plenum Press; 1998.
JCPDS—International Center for Diffraction Data; 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stefanescu, M., Stoia, M., Stefanescu, O. et al. Obtaining of Ni0.65Zn0.35Fe2O4 nanoparticles at low temperature starting from metallic nitrates and polyols. J Therm Anal Calorim 99, 459–464 (2010). https://doi.org/10.1007/s10973-009-0168-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0168-3